Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

In the life of a cell, just as in the course of a person’s life, there are times that call for a break in the routine; a moment to run a “self-check” to review the state of one’s internal health and settle on a course of action. When the genetic material of the cell is damaged, the cell needs to assess the extent of that damage and decide whether to turn on the DNA repair machinery or commit suicide to prevent the possible development of cancer. If this crucial self-evaluation stage is skipped, wrong decisions are likely to result.
The process of self-checking, decision making and action is managed by a crack team of proteins. Depending on the circumstances, certain members of this team may be given more than one assignment. In research published in the journal Cell, Dr. Atan Gross and his research team in the Biological Regulation Department at the Weizmann Institute showed that one prominent protein called BID lives the life of a double agent. BID plays two different roles: one in the chain of events that leads to cell suicide, and another in the chain that ends in survival. How is this one protein able to perform two, seemingly opposed missions?
In its better-known role as a regulatory protein in the suicide chain, BID begins to act when an enzyme, caspase-8, cuts a piece off the protein, leaving a smaller protein, tBID, that is primed and ready to carry out its assigned task in the cell’s suicide.
Gross and his research team began to learn of the protein’s double role when they asked what happens to the suicide chain if BID does not undergo cleavage. To answer this question, they inserted into embryonic mouse cells a gene that encodes for a mutant BID protein, one that resists cutting by the caspase-8 enzyme. To their surprise, the mutant, uncut BID was still activated. Gross and his team then tracked the activities of this protein and soon discovered that BID hooks up with another protein, ATM, a “player” for the anti-suicide side. ATM is a crucial manager of the DNA repair process in the cell nucleus; its role is so vital we can’t survive without it. It is called into action in response to toxins that severely damage DNA and cause breaks in both strands of the double-stranded chain. ATM, in turn, recruits a whole emergency crew to join in the rescue effort. Postdoctoral fellow Dr. Rachel Sarig, a member of Gross’s research team, found that along with the rescue workers, some of which science has not yet identified, ATM activates the full, uncut version of BID. If this is indeed the case, BID might play an important role in the chain of events that leads to DNA repair and cell survival.
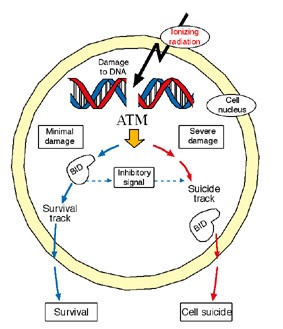
What is this role? To continue the investigation, research student Iris Kamer examined how the cell life cycle was managed in embryonic mouse cells lacking the gene for BID. These cells were exposed to a DNA-breaking toxin. The result: The BID-deficient cells skipped the critical self-check stage. Only when the scientists reintroduced BID into the cells did they take a break in the cycle to run a self-check.
The researcher’s investigations into the activities of this double agent revealed further insights into its whereabouts and dealings. They refuted current thinking, which maintains BID resides only in the body of the cell, showing that it also frequents the nucleus. The research team, which included Dr. Yehudit Zaltsman, Dr. Hagit Niv and research students Galia Oberkovitz, Limor Regev and Gal Haimovich, also found that BID can postpone the decision to commit suicide by a few hours, granting the cell’s DNA repair machinery a chance to undo the damage (a process in which BID might play yet another role). Although such a delay may seem inconsequential, pausing to seize the chance to preserve cell life rather than rushing into a decision to commit suicide may have some advantages.
When the cell is improperly managed, the upshot might be unsound decisions that could lead, for example, to the replication of damaged DNA or a delay in cell death – mistakes that can result in uncontrolled cell proliferation and the development of cancer. Gross hopes that the findings of his research team will contribute to a better understanding of cancer progression and the resistance of some tumors to chemotherapy and radiation, as well as helping to explain genetic diseases in which ATM is absent or has been inactivated.
Dr. Atan Gross’s research is supported by the Y. Leon Benoziyo Institute for Molecular Medicine; the Dolfi and Lola Ebner Center for Biomedical Research; the David and Fela Shapell Family Center for Genetic Disorders Research; the Willner Family Center for Vascular Biology; the Jeans-Jacques Brunschwig Fund for the Molecular Genetics of Cancer; the Harry and Jeanette Weinberg Fund for Molecular Genetics of Cancer; the Louis Chor Memorial Trust Fund; the Abisch Frenkel Foundation for the Promotion of Life Sciences; la Fondation Fernande et Jean Gaj; la Fondation Raphael et Regina Levy; and Mr. and Mrs. Stanley Chais. Dr. Gross is the incumbent of the Robert Armour Family Career Development Chair.