Every country has borders and – no less important – border crossings where goods and people can enter and leave. In the world of the cell, the cellular organelles act like little independent states: each conducting its specialized, vital processes within its borders while at the same time ensuring the flow of information and materials from one to the other. The recent discovery of a new “border crossing” between two organelles that were not even known to have “diplomatic relations” sheds new light on the ways that the organelles work together in a “federated state.”
The trans-border business of organelles can be conducted through couriers – secreted proteins that go from one to the other. But some types of passage require physical contact – to move materials from one to the other, for example. This takes place at specific contact points located around the border of the organelle. The first such contact point was identified over three decades ago, and new ones are still being discovered.
Dr. Maya Schuldiner of the Molecular Genetics Department says that every new discovery of this sort adds to the growing picture of the interrelations between organelles, the movement of information and materials within the cell, and obstacles that can cause disease.
In her postdoctoral research at the University of California, San Francisco, in the group of Prof. Jonathan Weissman, Schuldiner had worked with the team in Prof. Peter Walter’s lab that discovered a contact point between two of the cell’s main organelles. It connected the mitochondria – the cell’s power plants – with the endoplasmic reticulum in which, in addition to its function in readying proteins for secretion outside the cell, fats are produced. Together, these two organelles take up close to a third of the cell’s volume. They cling together at their contact points through “zipper proteins,” allowing fat molecules from the endoplasmic reticulum to pass into the mitochondrion, which uses them to build membranes.
The scientists surmised that if there were damage to the zipper proteins, the passage of fats, and thus the membrane structure of the mitochondria, would be impaired. But to their surprise, no such effect ensued. The conclusion was that the mitochondria had an as-yet-undiscovered contact point for obtaining fat.
Dr. Yael Elbaz-Alon, a postdoctoral fellow in Schuldiner’s lab, took up the challenge of finding that unknown contact point. Her starting theory was that if the flow of material (in this case fat) to the mitochondria remains constant when one of two available sources dries up, crossing points for the other one must double in order to keep up with demand. So the scientists looked at yeast cells – which have a somewhat manageable 6,200 proteins – and disabled these proteins one by one, looking for any case in which the known contact point, highlighted with a fluorescent marker for visibility, would double its activity. The lab’s robotic system enabled the researchers to conduct this test for all the proteins automatically.
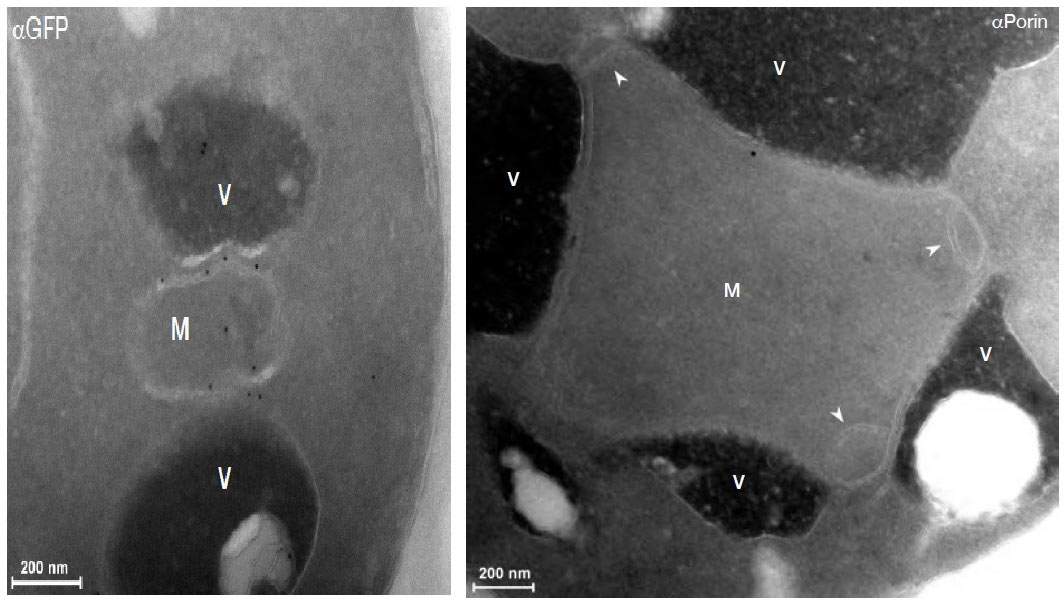
This strategy turned up four proteins that, when damaged, cause the known contact points to multiply. The team then looked at the locations of these four proteins in the cell. And, indeed, one of them appeared on the mitochondria’s borders – but in an unexpected place. This protein formed a border crossing to another organelle – the vacuole (similar to the lysosome in humans). This is a cellular recycling plant that naturally contains large quantities of fat molecules. It appears that the mitochondria have two sources of fat – freshly produced fat from the endoplasmic reticulum or recycled fat from the vacuole – and two types of border crossings to match.
The research, which recently appeared in
Developmental Cell, not only revealed a new border crossing, but a new border as well. The new contact point, which has been named vCLAMP, has evaded detection until now, says Schuldiner, because “in normal cells there are very few of them. Only when the previously-known border crossings were damaged did the numbers of the second swell to the point where they could be easily seen with an electron microscope. In fact, when these contact points were forced to take over, we also saw unusual clumps of mitochondria surrounded by vacuoles.”
Evidence for the existence of vCLAMP has been found in humans, meaning that this phenomenon is an important one that has been preserved throughout evolution. Schuldiner’s research group plans to study this phenomenon in depth to understand just how important it is.
Also participating in this research were
Prof. Tony Futerman of the Biological Chemistry Department and Eden Rosenfeld-Gur, a research student in his group; Dr. Vera Shinder of the Electron Microscopy Unit; and Dr. Tamar Geiger of the Sackler Medical Faculty of Tel Aviv University.
Dr. Maya Schuldiner's research is supported by the Foundation Adelis; the Georges Lustgarten Cancer Research Fund; the Dora Yoachimowicz Endowed Fund for Research; the Berlin Family Foundation; Roberto and Renata Ruhman, Brazil; the European Research Council; and Karen Siem, UK.
Prof. Anthony H. Futerman's research is supported by the Nella and Leon Benoziyo Center for Neurological Diseases, which he heads; the M.D. Moross Institute for Cancer Research; the Carolito Stiftung; and the Rosetrees Trust. Prof. Futerman is the incumbent of the Joseph Meyerhoff Professorial Chair of Biochemistry.