Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Nature supplies us with dramatic lessons on the importance of structure. Alternate structural versions of chemical elements, called allotropes, are sometimes so disparate that it is hard to believe they have the same composition. The allotropes diamonds and graphite, for example, are both made exclusively of carbon, differing only in the arrangement of the carbon atoms.
As reported in Science, a team headed by Prof. Rafal Klajn of the Organic Chemistry Department of the Weizmann Institute of Science has now created allotropes that don’t exist in nature: Rather than being distinct on the atomic scale, these materials differ in their nanostructure – that is, on a scale 10 to 100 times the dimensions of individual atoms. Such nanoallotropes – that is, allotropes of nanomaterials – may help create ultra-small electronic devices and find other industrial applications.
By altering the ratio, the scientists could precisely control the nanoallotrope’s structure
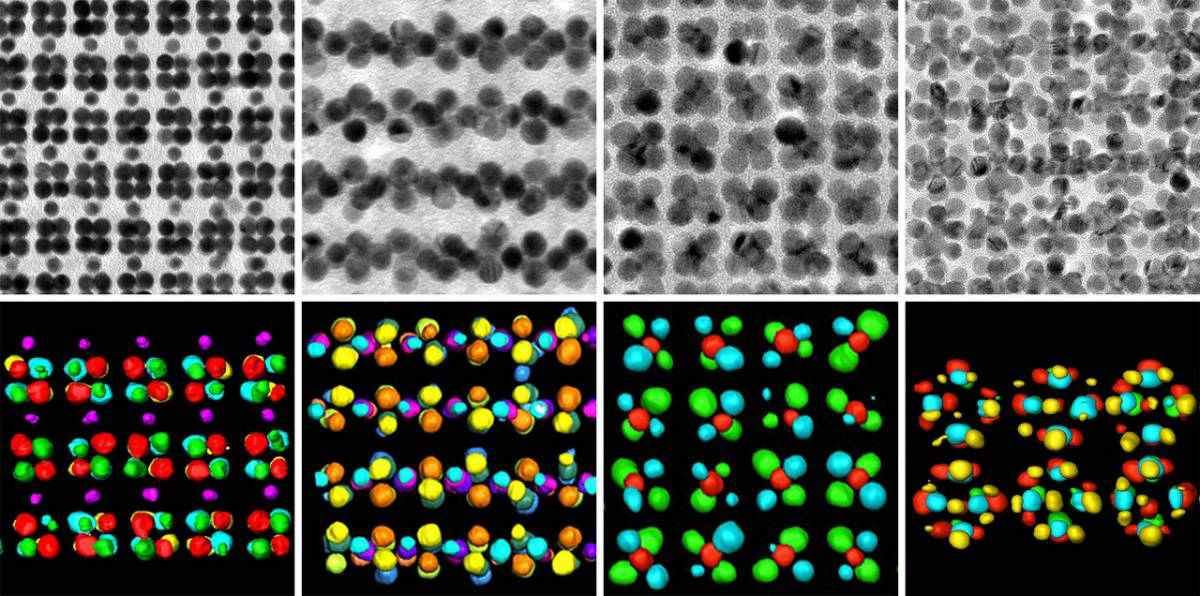
Together with postdoctoral fellow Dr. Thumu Udayabhaskararao and other researchers from Weizmann – as well as from the United States, Spain and Belgium – Klajn created a two-step method for generating nanoallotropes. In the first step, the researchers mixed nanoparticles of gold with those of iron oxide in a solution, causing them to self-assemble into a crystal-like film on top of a liquid. In the second step, the scientists exposed this film to the beam of an electron microscope and immersed it in acid, which removed the iron oxide, leaving a lattice of gold nanoparticles alone.
The researchers found that by mixing the two types of nanoparticles in different ratios to one another, they could obtain different lattices. Every time they then removed the iron oxide, the result, in turn, was a pure lattice of gold nanoparticles with a different internal architecture – in other words, a different nanoallotrope of gold. By altering the ratio, the scientists could precisely control the nanoallotrope’s structure.
The nanoallotropes were found to be substantially different from one another, as is the case with classical allotropes. They differed, for example, in their optical properties and could also differ in the way they conducted a current of electrons. Although the study was performed with nanoparticles of gold, it could be extended to other materials as well.
The new method may help fabricate electronic devices that are smaller than existing ones. At present, the circuits that channel the electric current in a desired direction are produced by means of lithography using an electron beam – a technique that is expensive and limited in resolution. Fabricating the circuits by means of nanoparticle self-assembly may make it possible to greatly decrease the costs while shrinking the devices more than is feasible with today’s methods.

Among numerous other applications, nanoallotropes may help create porous films with a mesh of predefined holes for the purpose of filtration, or substrates that will catalyze chemical reactions – for example, for destroying contaminants in order to purify water. “Our method makes it possible to design stable, chemically robust nanoallotropes with a predetermined structure, and it can be applied to numerous metals and semiconductors,” says Klajn.
Taking part in the study were Drs. Ronit Popovitz-Biro and Lothar Houben of Weizmann’s Chemical Research Support Department; Dr. Thomas Altantzis and Prof. Sara Bals of the University of Antwerp, Belgium; Dr. Marc Coronado-Puchau, Dr. Judith Langer and Prof. Luis M. Liz-Marzán of the Center for Cooperative Research in Biomaterials, Spain; Prof. Lela Vuković of the University of Texas at El Paso, USA; and Prof. Petr Král of the University of Illinois at Chicago, USA.
Prof. Rafal Klajn's research is supported by the Helen and Martin Kimmel Center for Molecular Design, which he heads; the Yeda-Sela Center for Basic Research; the Rothschild Caesarea Foundation; the European Research Council and the Israel Ministry of Science and Technology .