Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Crystallization is a very basic chemical process: School children can witness it with their own eyes. But scientists had not, until now, been able to observe this process on the molecular level – that is, the instant in which molecules overcome their tendencies to float individually in a liquid solution and take their place in the rigid lattice of a solid crystal structure. Researchers at the Weizmann Institute of Science have, for the first time, directly observed the process of crystallization on the molecular level, validating some recent theories about crystallization, as well as showing that if one knows how the crystal starts growing, one can predict the end structure.
The research took place in the lab of Prof. Ronny Neumann of the Weizmann Institute’s Organic Chemistry Department. Neumann explains that in order to bond to one another, the molecules must overcome an energy barrier. “The first theories about crystallization date back to the late 19th century,” he says. “The prevalent theory since that time has been that chance contacts between molecules leads to bonding, eventually creating small clusters that become nuclei for larger crystals to grow. But the molecules, which move randomly in solution, must be aligned properly to crystalize. In recent years, researchers have begun to think that this process might present too high an energy barrier." Theories proposed in the past few decades suggest that if the molecules were to congregate together in a so-called dense phase, in which they aggregate into a sardine-like state – close together but unorganized – and then crystallize from this state, the energy barrier would be lower.
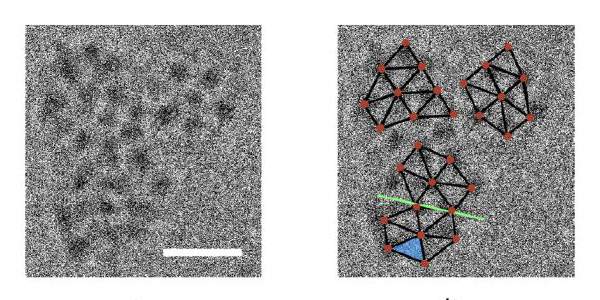
To test the theories, Neumann and PhD student Roy Schreiber created large, rigid molecules and froze them in place in solution. They then placed the frozen solution under an electron microscope beam that warmed up the mixture just enough to allow some movement, and thus interactions between the molecules. Adjusting the makeup of the solution by adding different ions enabled the scientists to produce crystallization with and without dense phases; for the first time, aided by Drs. Lothar Houben and Sharon Wolf of the Electron Microscopy Unit, they were able to observe dense phases forming and subsequently transforming into crystal nuclei.
While both states yielded crystals, the experimental results showed that when dense phases form, the energy barrier to formation of an orderly, crystalline arrangement of molecules is, as the theory predicted, lower.
We have really observed an elementary event in the world of chemistry
The scientists also found that the growth arising from dense phases results in larger, more stable crystal nuclei. In addition, they discovered that the arrangement of molecules in fully grown crystals, which they determined by X-ray crystallography with the aid of Dr. Gregory Leitus of Chemical Research Support, was in good agreement with that in the small clusters of just a few molecules in the original nuclei. “This means that the forces and factors that determine the process are constant throughout the growth of the crystal,” says Neumann.
“We have really observed an elementary event in the world of chemistry,” says Neumann. “The findings are also leading us into new inquiries in this area, looking at the effects and significance of dense-phases on chemical reactivity.”
Also participating in this research was a group led by Prof. Josep Poblet, Dr. Jorge Carbó and PhD student Zhong-Ling Lang of the University of Rovira i Virgili, Tarragona, Spain, who assisted in the calculation of the motion of molecules in solution.
Prof. Ronny Neumann's research is supported by Dana and Yossie Hollander, Israel; and the Bernice and Peter Cohn Catalysis Research Fund. Prof. Neumann is the incumbent of the Rebecca and Israel Sieff Professorial Chair of Organic Chemistry.
Crystallization from solution by molecular attachment (higher energy pathway)