Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Energy storage capacity can be a critical feature in smartphones, laptops, electric vehicles and a broad range of other battery-powered devices. One common solution is to use supercapacitors, an emerging class of high-power units that store charge in electrodes made from porous materials. The pores – the nano-sized nooks and crannies – vastly increase the surface area available for storing charged particles. But they also slow down the pace at which the electrodes can accept and deliver charge. As reported recently in Nature Communications, researchers at the Weizmann Institute of Science have developed a method for measuring the speed at which charge is accepted or delivered by an individual nano-pore.
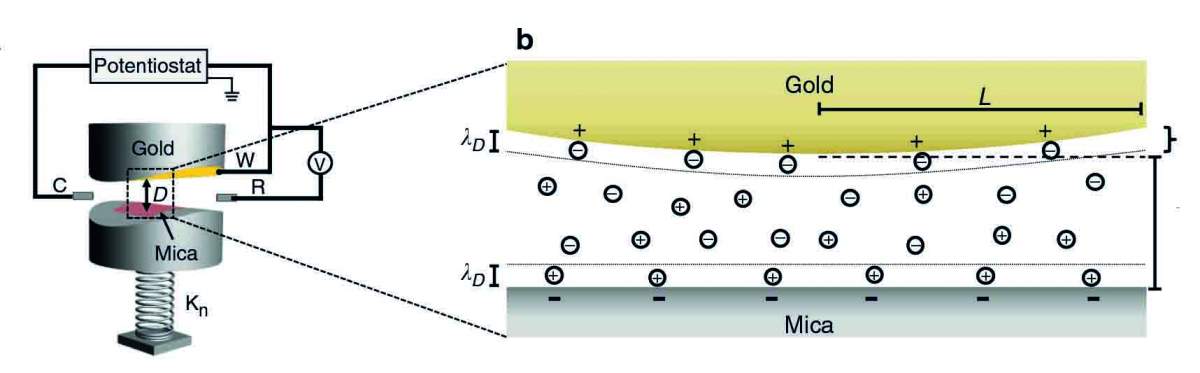
Batteries and supercapacitors were initially far from the minds of the Weizmann scientists. Rather, Prof. Jacob Klein of the Materials and Interfaces Department and his colleagues wanted to know what happens to forces acting between two surfaces on the nano-scale when the electric potential of one of them is altered. The researchers designed an apparatus in which two round super-smooth plates ‒ one gold, the other made of an insulating mineral called mica ‒ faced each other across a tiny gap. This setup created a sort of nano-slit defined by the width of the gap ‒ about 50 nanometers (billionths of a meter) long and a diameter of around 100 microns (millionths of a meter). The scientists immersed the plates in a solution containing charged ions, changed the potential of the gold surface in a sudden manner and monitored the electrostatic force – in which charged surfaces repel or attract one another – resulting from the change.

The way in which the force evolved told the scientists a great deal about the charging dynamics within the nano-gap. Among other things, the experiment enabled them to measure the time it took the surfaces to acquire the new charge and the time it took them to lose it.
“That was when we realized that measuring the forces across our nano-slit makes it possible to measure the charging time of a nano-pore in an electrode – that is, the time it takes the charge-transporting ions to enter or exit this pore,” Klein says. Building on earlier research in his lab by former student Dr. Liraz Chai, he conducted the study with then graduate students Drs. Ran Tivony and Gilad Silbert, departmental colleague Prof. Sam Safran and Prof. Philip Pincus of the University of California, Santa Barbara.
In the experiment, it took about one second for the ions carrying an electric charge to enter or exit the nano-slit. Various parameters were found to affect the duration of this entry and exit. For example, the charging occurred much faster when the slit was wider and when the concentration of ions in the solution was increased. Conversely, the charging took longer when the slit was narrower. These findings suggest that the measurements performed in the study can help direct the development of improved porous materials. That is, it can enable experimenters to check which parameters need to be changed in a given material so as to speed up the charging and delivery of the charge. Such experimentation can help improve porous electrodes for use not only in existing technologies but also in such emerging applications as water desalination and the extraction of renewable energy from salt solutions.

Prof. Jacob Klein's research is supported by the Charles W. McCutchen Foundation; the Edmond de Rothschild Foundations; Simon and Olga (Golde) Picker; and the European Research Council. Prof. Klein is the incumbent of the Hermann Mark Professorial Chair of Polymer Physics.