Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
How do some bacteria build impregnable fortresses large enough to shelter whole “cities” and simultaneously migrate to distant organs or to the opposite side of a lab dish? The answer, according to a new study led by Weizmann Institute of Science researchers, is that the very glue that holds these shelters together also serves as a signal to some of the bacteria to leave the home colony and start migrating. In addition to providing possible new targets for treating certain kinds of bacterial infections, including some that are antibiotic-resistant, the research may provide insight into the development of such other diseases as Alzheimer’s and cancer.
Prof. Ilana Kolodkin-Gal of the Weizmann Institute of Science’s Molecular Genetics Department researches bacterial shelters called biofilms, both their means of construction and the ways in which the bacteria collaborate to build them. It was generally believed that the rigid biofilms involved a trade-off, limiting the bacteria’s mobility in return for protection. “Until quite recently, it was thought that once a bacterium was enclosed within a biofilm, the genes and structures for mobility were frozen. But we have noticed that biofilms don’t just spread from the edges – their residents can move quite a distance from the original colony, and they seem to do it in a way that affords them the same protection they get from staying put within the biofilm. We began this research by asking how they manage to have the best of both worlds – rigid protection, as well as mobility.”
Motile cells move forward during engulfment, followed by matrix producers
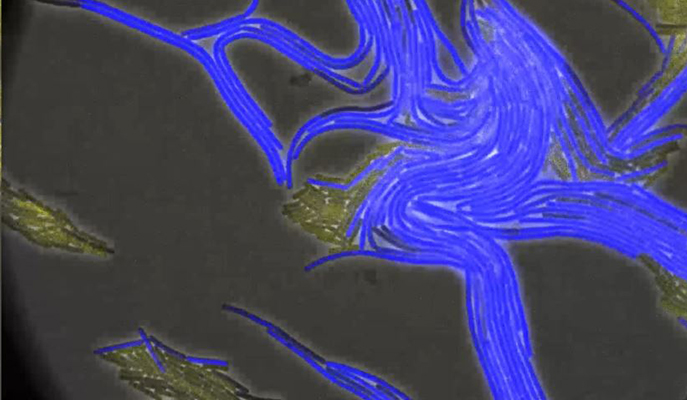
Kolodkin-Gal, together with graduate student (now Dr.) Nitai Steinberg and Dr. Alona Keren-Paz from her group, and Dr. Tsviya Olender of the Molecular Genetics Department joined forces with the groups of Prof. Diego Romero, from the Institute of Malaga, Spain, and Prof. Sven van Teeffelen, of the Pasteur Institute, Paris. Together they created imaging methods, based on those used in the Pasteur Institute that would enable them to visualize the biofilm-forming bacteria in real time and in such extreme detail that they were able to observe the process in which the thin fibers that tie one bacterium to another were spun.
The substance making up those fibers, or the glue in the biofilms, is amyloid – almost exactly the same amyloid that is infamous for creating tangled structures in the brains of Alzheimer’s patients. Amyloids are common in nature – so common that no one had paid much attention to the part they play in holding biofilms together. Kolodkin-Gal and her team came upon the role that amyloids play in migration through a process of elimination – they created mutant bacteria that were missing various genes needed to either migrate or form biofilms, and they were puzzled to find that some that had trouble forming the biofilms also had trouble migrating. Further elimination experiments and real-time filming under a powerful microscope confirmed that a particular amyloid involved in construction also served, to certain bacteria near the biofilm’s surface, as a signal to start up the migration machinery. This was the first time, says Kolodkin-Gal, that anyone had observed such amyloids – which are formed by a subset of the bacteria – being made in real time; and the insights provided by these videos enabled them to trace the biochemical pathway leading from amyloid formation to migration.
The imaging showed these bacteria moving off in tiny clusters that are like miniature biofilms in themselves. Some of the bacteria in these clusters specialize in building, others in moving. The amyloids – sticky and flexible to begin with – create tethers that keep each of the clusters moving together. This group formation protects them from things such as roving immune cells that might swallow lone bacteria whole. When it arrives at a free location, this bacterial team is all set to begin creating the next biofilm on a new surface.

While the hard biofilm structures can be impenetrable to antibiotic drugs, Kolodkin-Gal and the team discovered that the stress the bacterial cell experiences when it produces amyloids also has the effect, by activating the secretion stress machinery, of increasing its ability to withstand the drugs.
One conclusion of the research is that antibiotics might work better if anti-amyloid treatments were administered as well. Such drugs have already been developed to dissolve the amyloid tangles in Alzheimer’s, and while these have turned out to have little effect on the degeneration of cognitive abilities in patients, they could potentially break apart biofilms, reducing antibiotic resistance and preventing the bacteria from spreading. Although the bacteria in Kolodkin-Gal’s lab are not pathogenic, they are closely related to infectious bacteria that form biofilms on heart valves, the urinary tract, the stomach and the lungs, so these findings could have immediate relevance for these types of infections.
Expression of the flagellin GFP reporter in colonies over time
For Kolodkin-Gal, this research has answered one outstanding question – how biofilm-forming bacteria migrate – but it has raised a number of larger research questions. For one, cancer cells have been found to migrate in a similar manner, tethered together in small clusters, and she thinks further research might find that other kinds of cells also use this kind of “tied-together teamwork” to migrate. And there have been a few recent studies hinting at a puzzling link between bacterial infections and the subsequent onset of Alzheimer’s. Kolodkin-Gal thinks that the amyloids made by the bacteria could be the missing link in this research. “In the brain, these structures are accidental, but with bacteria, they are intentional and highly-controlled,” she says. “It is really quite ingenious.”
Prof. Ilana Kolodkin-Gal's research is supported by the Kekst Family Institute for Medical Genetics; the Angel Faivovich Foundation for Ecological Research; the estate of Helen Nichunsky; the estate of Raymond Lapon; and the estate of Albert Engleman. Prof. Kolodkin-Gal is the incumbent of the Rowland and Sylvia Schaefer Career Development Chair in Perpetuity.