Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
At any one time, some of the blood-forming stem cells in the bone marrow are living quiet, sheltered lives, while others are busy differentiating into blood cells and moving into the bloodstream. How does the bone marrow offer protection to some of its stem cells while at the same time shoving others out of the door? The answer, according to Weizmann Institute of Science research conducted in the lab of Prof. Tsvee Lapidot of the Institute's Immunology Department in collaboration with Harvard Medical School, the Max-Planck Institute and the Weizmann Institute group of Prof. Guy Shakhar, lies in distinct types of blood vessels that run through the bone marrow.
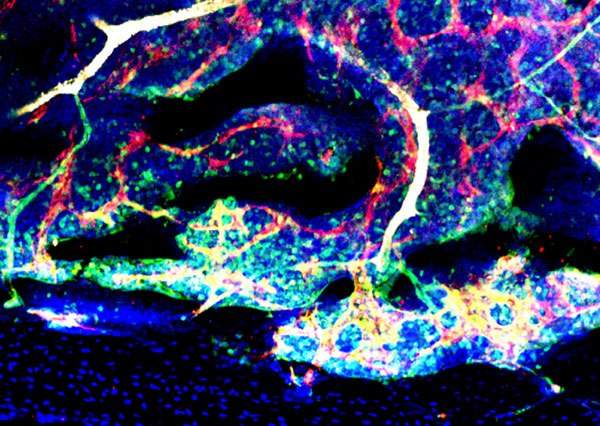
Lapidot and postdoctoral research student Dr. Tomer Itkin knew that the special niches the blood-forming stem cells occupy are clustered around the bone marrow blood vessels. But no one had yet asked which blood vessels these were. In their study, the researchers found that the two major types of small vessel in the marrow, arterioles and sinusoids, have their own spaces: Arterioles stick mainly to the outer ring near the bone, while sinusoids run all over the marrow. Further investigation revealed that stem cells residing in niches near the aterioles had low metabolic states, while those near the sinusoids had varying levels of molecules called reactive oxygen species (ROS), which among other things cause the cells to differentiate and migrate into the bloodstream.
The scientists further discovered that arterioles tended to be well sealed whereas the sinusoid walls had leaky pores, and the team found these to be the exclusive sites for cellular trafficking. Why is this important? Along with the cell movement, blood plasma can penetrate from the peripheral blood into the marrow. When the team exposed quiescent stem cells to plasma, these began to differentiate and even to die as their ROS levels rose. “The sleepy stem cells don’t like blood plasma,” says Itkin. “A little plasma irritates them into differentiating and enhances their motility, but we found that too much can lead to their death.”
The sleepy stem cells don’t like blood plasma
Using genetic and biochemical methods on mouse blood vessels, the scientists altered the permeability of the vessel wall to see if they could affect the fate of the stem cells residing in the bone marrow. Indeed, when blood vessel pores closed up, the pool of stem cells increased, as the cells divided without differentiating. When pores were opened, the bone marrow pool of stem cells was depleted as they either differentiated and moved into the bloodstream or died.
These findings have crucial implications for stem-cell transplantation. For example, they point to ways of helping donor stem cells to move into the bloodstream to increase the harvest yield for transplantation or for ensuring that the transplanted cells make it all the way and engraft to the proper niches in the bone marrow. “Ideally, you would increase the vessel permeability just long enough for the cells to go from the blood into the bone marrow, and then close things up before the cells can get out again or die,” says Lapidot.
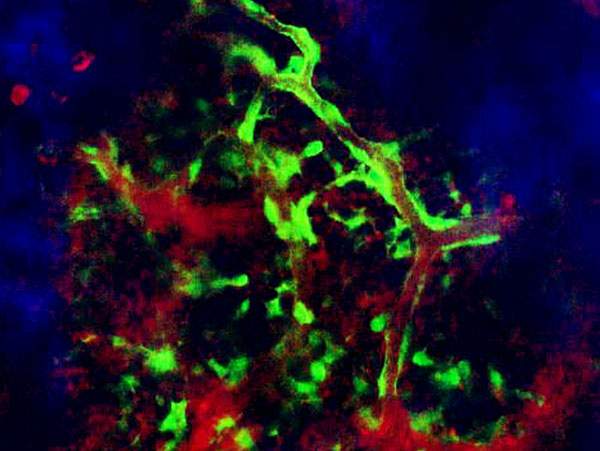
The team noted that the other stem cells in the bone marrow – bone-forming stem cells – are also regulated by the blood vessels’ permeability. Since many adult stem cells are found near blood vessels in other organs, the researchers think that the blood-vessel-barrier system may be common in our bodies, keeping the stem cell pools safe while enabling new mature cells to replace the old and damaged ones.
Prof. Tsvee Lapidot's research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research, which he heads; the Benoziyo Endowment Fund for the Advancement of Science; the Leona M. and Harry B. Helmsley Charitable Trust; the Adelis Foundation; and the Dr. Beth Rom-Rymer Stem Cell Research Fund. Prof. Lapidot is the incumbent of the Edith Arnoff Stein Professorial Chair in Stem Cell Research.