Chemistry was the first discipline established at the Weizmann Institute, and its chemists continue to make their mark in areas ranging from the elucidation of such basic structures of life as the ribosome (the body’s protein factory) to the development of innovative industrial processes.
Among the everyday products and processes emerging from Weizmann labs are enzymes tied to synthetic resins that aid in manufacturing synthetic antibiotics, corn syrup, and other materials. Brittle ceramic superconductors have been endowed with metal-like ductility, thus increasing the possibility of their use as frictionless ball bearings. Inorganic materials containing soccerball-shaped structures, first identified at the Institute, may one day serve as novel solid lubricants. Tailor-made iron-binding organic molecules have been shown to hold potential as minute computer switches.
The familiar photochromic materials that reversibly darken when exposed to sunlight are the result of a Weizmann Institute discovery many years ago. A new development may allow relatively heavy eyeglass lenses to be replaced by lightweight plastic ones with a self-tinting coating.
The Quest for New Materials
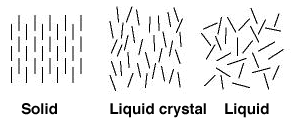
A new shape for liquid crystals
A liquid crystal is an intermediate state between a solid crystal, where all the constituent molecules are arranged in the same direction, and a liquid with molecules pointing in random directions. Weizmann Institute scientists, in collaboration with scientists from the Max Planck Medical Research Institute in Heidelberg, were the first to demonstrate that it is possible to use specially produced pyramidal molecules to create a new group of liquid crystals composed of cone-shaped molecules. Until this discovery, the only liquid crystals known were composed of cigar- or coin-shaped molecules.
The properties of the liquid crystals developed by Weizmann scientists were investigated by various methods, including nuclear magnetic resonance. Aside from expanding the variety of materials exhibiting liquid crystallinity, these studies led to the discovery of a correlation between the organization of liquid crystals and the structure of their constituent molecules.
It's all on the surface
Which material in a mixture tends to concentrate near the surface and which concentrates chiefly in the center? This question is of extreme importance since material concentrating close to the surface will determine many of the mixture's properties, such as its smoothness, friction with its surroundings, the ability to absorb or reflect various radiation, and whether it may be easily cleaned.
Weizmann Institute scientists discovered that when a mixture contains two chemically similar polymers, the more flexible one concentrates near the surface, creating layers. Comprehension of the way materials organize themselves in mixtures may help in planning diverse materials and industrial processes in the future.
Here comes the rain
Arid countries sometimes use ice seeders to increase rainfall. The technique is based on the fact that although ice becomes water when it reaches a temperature of 0°Celsius (32°Fahrenheit), water can exist at less than -20°C (-4°F) and even -40°C (-40°F) without it turning into ice. In order for rain to fall, ice crystals must form in the water vapor in clouds. These crystals descend (due to their relatively high weight) and while doing so, melt, turning into rain drops. However, when the cloud vapor cools without ice crystals being formed, no rain falls.
To increase rainfall, it is possible to seed clouds with different materials to accelerate the formation of ice crystals. Today, the most widely-used ice seeder is silver iodide which freezes water vapor at a temperature of less than -8°C (-17.6°F). Now Weizmann Institute scientists have identified materials that may be used to accelerate the creation of ice crystals at higher temperatures. The scientists discovered that some long-chain alcohols freeze water vapor at a temperature higher than -8°C.
The scientists found that the length of an alcohol molecule and its pairing or nonpairing (determined by the number of carbon atoms in it), fulfill a decisive role in its ability to freeze water vapor. The longer the alcohol carbon chains, the higher the temperature at which it freezes water. In addition, nonpaired alcohols can freeze water at temperatures just below 0°C, while paired alcohol can freeze it at -8°C.
Molecular brushes
Weizmann Institute scientists developed a new field of study regarding the special properties of molecular brushes formed, for example, when long molecules are grafted to a surface. These molecular brushes repel each other, giving stability to substances such as ink, milk, perfumes and liquid drugs.
Molecular brushes minimize friction
Friction is the main cause of decreased efficiency in machinery. Regular lubricants usually contain small molecules that produce thin organic films, but they tend to solidify under pressure and reduce friction only to a limited extent. Now Weizmann Institute scientists have developed a means to cut friction by 1,000-fold (or more), based on long, flexible molecules, or polymers.
Polymer brushes are created by binding the ends of polymers onto two rubbing surfaces in a fluid. The free ends of the molecules protrude into the fluid between the surfaces, like the bristles of a brush. These polymer brushes penetrate each other only minimally, even when the surfaces are pressed against each other with great force, and thus can slide easily past each other. This means they constitute a highly effective way of reducing friction.
Artificial peptides that mimic nature
Weizmann Institute scientists discovered that when certain hydrophobic amino acids are dissolved in water, the molecules floating on the aqueous surface divide into two groups based on their different structural direction (whether they are left- or right-handed). The scientists are now trying to exploit this phenomenon to produce artificial peptides (short proteins) that are similar to natural peptides formed from amino acids with the same structural direction.
Free-flowing oils need long-chain molecules
How and why are cholesterol lumps, which may block blood vessels, formed? What are the initial stages that occur when water turns into a layer of ice? What can be done to prevent the formation of wax plugs in machinery's oil pipes during freezing weather? All these questions focus on one important natural process: the formation of crystals. Comprehending the laws controlling this process should provide researchers with the ability to accelerate or delay it, or even prevent crystallization when it is unwanted.
Weizmann Institute investigators arrived at new conclusions regarding these laws. The researchers concentrated on the formation of wax crystals and discovered that molecular chains containing 36 carbon atoms, which sink to the bottom of a container or accumulate on a pipe wall, are arranged in a layer one molecule thick and form a two-dimensional crystal. In contrast, shorter molecular chains form sediments composed of many layers, which cause the rapid formation of wax plugs. For example, a chain of 23 carbon atoms creates an initial foundation 20 molecules thick.
The researchers irradiated thin layers of wax that had formed on the surface of water with an extremely powerful X-ray beam. Based on the way the beam was diffracted, the investigators worked out the exact structures of the wax crystals formed by sedimentation of different substances in the oils. They then demonstrated that certain alcohols prevent the interlayer accumulation of carbon molecules while controlling the crystallization process.
The improved understanding of these early stages of solidification has led to new methods for separating optical isomer mixtures (molecules that are a mirror image of each other). These methods are being used in the pharmaceutical industry and in developing new organic materials for use in seeding clouds to increase rainfall.
How the mollusk builds its shell
Mollusks build their shells like a composite material- a structure based on a matrix in which one component (usually flexible) is filled by another substance (usually hard). Mollusk shells are built on a matrix of proteins filled with calcium carbonate which can crystallize into different structures. This allows one layer of a shell to be constructed of aragonite crystals while the other is calcite. These two mineral forms are identical chemically and similar structurally, but very different in external form.
Weizmann Institute scientists found that a mollusk controls whether the calcium salt will crystallize as an aragonite or a calcite by changing the proteins it secretes to form the matrix. They did this by assembling in the organic framework in which crystals form in a test tube and then adding specific proteins that induced either aragonite or calcite to form. These findings may contribute to the future development of new methods for producing improved artificial composite materials for applications.
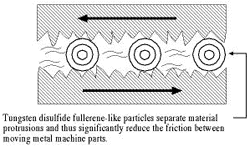
Novel lubricant reduces wear and tear
Weizmann Institute researchers identified new forms of the inorganic material, tungsten disulfide, with a closed structure resembling a soccer ball and nanotubes. Until this discovery, many scientists assumed that only carbon could be arranged into this shape (known as fullerenes and nanotubes). Following the Weizmann study, it became apparent that fullerene-like structures can exist in various inorganic materials, including semiconductors and semimetals. In further studies, Institute scientists demonstrated that fullerene-like tungsten disulfide is an especially effective lubricant (2.5 times better than the alternatives, such as oil-based liquids with various solid lubricants as additives); it reduces the wear and tear on moving machinery by five-sixths when compared with regular lubricants.
The Weizmann Institute discovery has led dozens of groups of scientists in different parts of the world to begin research into the field of inorganic fullerene-like materials.
When semiconductors become insulators
In the 1950s, Weizmann Institute scientists determined the electronic band structure of the semiconductor materials silicon and germanium. The scientists relied on measurements of the Hall effect and the influence of magnetic fields on electric resistance. These studies made a decisive contribution to the development of semiconductor technology on which modern electronics is based.
The leap from chemistry to life
One of the important phenomena in chemical evolution and the life creation process is chirality: the existence of molecules with an identical chemical composition but differing from one another by their spatial structure, so that one of the molecules is a mirror image of the other (like the similarity and the difference between our right and left hands). It is impossible to superimpose one of these molecules on the other (just as it is impossible to superimpose our hands).
The chemical reactions that occurred prior to the existence of life led to the formation of equal quantities of right- and left-handed molecules. In contrast, in biological systems, the different types of molecules (such as amino acids) will have a common chirality (or "handedness"). The question is: How did a world of inanimate materials where two types of chiral molecules existed happily together, change into a world where molecules have a clearly preferred direction?
Weizmann Institute scientists developed a method that represented such a change, based on the organizational process of molecules in crystals. This method led to a way of controlling the design of crystals and the way they grow, and may serve as an important tool in tailoring drugs.
Mysteries of the Molecule
The beginnings of nuclear magnetic resonance
In the 1950s, while the Weizmann Institute was still in its infancy, scientists constructed a high-resolution nuclear magnetic resonance (NMR) spectrometer, only the second such device to be built in the world. The machine was used for a pioneering study of chemical reaction rates under equilibrium conditions, as well as the study of molecule structure and behavior.
Throwing light on chemistry
The Weizmann Institute's scientists were among those who first laid the foundations of modern photochemistry. As early as the 1950s, scientists from the Institute studied the behavior of various molecules, particularly organic ones, under the influence of visible light rays, as well as ultraviolet light. This research, which focused on how molecules in a variety of situations absorb radiation, gave rise to important insights and the development of ways of carrying out a number of chemical reactions using light. Among other things, the Institute's scientists developed new ways of generating fluorinated compounds and various aromatics.
The future is in plastics
In the 1950s, Weizmann Institute scientists made important contributions to the development of methods for producing artificial rubber-like materials. The research was based primarily on the understanding of the properties of conjugated chemical bonds (in which carbon atoms in a molecule are linked by alternating single and double bonds) and their use in the manufacturing processes of rubber substitutes. These processes have been perfected over time, and today find application in the plastics industry.
Reactions in solid crystals
Weizmann Institute scientists found a clear link between the spatial organization of organic molecules in crystals, and the products obtained after a chemical reaction between these molecules in their solid state. This pioneering and original research, carried out in the 1950s and 1960s, led to the ability to plan, in advance, the organizational structure of molecules in crystals, and consequently, the nature of the chemical reactions which they are able to perform.
In this study, Institute scientists learned to exploit the properties of solid materials, in order to carry out chemical reactions that allow non-chiral molecules (those that are symmetrical, rather than having a left-handed or right-handed structure) to crystallize in a chiral crystal. The scientists produced chemical reactions between molecules in these crystals, and succeeded in obtaining products with a single clear-cut chirality.
The math and physics of molecules
Institute scientists developed a method for calculating the forces exerted by various molecule components on one another. This method, known as the consistent force field, makes it possible to characterize and describe molecules quantitatively, as well as to predict and calculate the binding energy that acts between their components. It also allows researchers to study the activity of various molecules in biological systems, such as how ions bind to molecules and how proteins are structured.
New imaging method reveals molecular structure
The spatial structure of a molecule is an important factor in determining how it functions chemically and physically. Weizmann Institute scientists have developed a new imaging method that can elucidate the structure of various molecules. The new technique provides direct geometrical pictures of single molecules, without requiring any prior hypotheses. This has already made it possible to determine several previously unknown molecular structures.
The scientists used a cold source that supplies negative molecular ions (molecules with one surplus electron) in their most stable form. These molecular ions are accelerated to extremely high speeds and only then bombarded with a laser beam. The result is a beam of high-energy neutral molecules in clearly-defined excited states. When the molecules pass through a very thin foil, the electrons which hold their atoms together are torn away. This means that the molecular atoms become positively-charged ions due to this deficiency of electrons. These positive ions repel each other and, as they try to get away, the ionic cloud of each molecule grows to a billion times its original size, enabling scientists to directly observe the molecules' structure.
The researchers also apply this method to examine the helical direction of molecules, to see if they have a right-handed or a left-handed helix-like structure. This property of left- or right-handedness in molecules is of great importance in industry, life sciences, and drug development.
The basis of antibiotic production
A novel approach for the industrial production of synthetic antibiotic preparations is based on vanguard research carried out by Weizmann Institute scientists, who developed a range of chemically-active polymers that are used for the preparation of various substances, including biologically-active peptides.
Using light to break the carbon bond
Weizmann Institute scientists have developed efficient, simple and cheap ways of using light to break the bonds between carbon and oxygen, nitrogen or sulfur. Today, these methods are a central tool in the chemistry of peptides, sugars, and phosphates in "molecular libraries" and in sequencing genes, for example, for the purposes of the Human Genome Project.
One application of the method is to bind very large numbers of DNA sections to a surface. This permits the rapid reading of a DNA sequence. The method may also be useful in the controlled release of drugs in the body, lessening the potential for side effects.
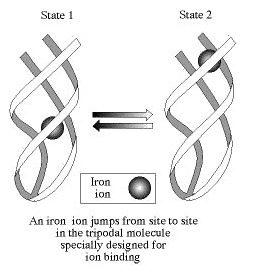
Organic switches
Organic molecules can be designed to perform a wide spectrum of specific tasks and Weizmann Institute scientists have been responsible for the synthesis of several of them. Examples include a family of molecules that have special sites for binding metal ions as part of their skeleton. These molecules may be used as metal ion scavengers in the fight against malaria and other parasites, or as transporting agents which deliver drugs to distinct locations in the body. This may reduce the current quantity of required drugs and their subsequent side effects.
The same kind of molecules may be used in another flourishing area, the field of computers. They may serve as molecular switches for computer memory banks, which will be much faster and occupy a much smaller space than the memory banks that are currently in use. Possible applications of the molecular switches are being evaluated jointly by the Weizmann Institute of Science and a large industrial firm.