Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Molecules mostly get depicted as static objects – for example, as balls held together with sticks, like children’s toys. The balls in these models represent the atomic nuclei and the sticks are the bonds created by the electrons; from the relative positions of these sticks and balls, we can assume a shape for the molecule. If we could build a more realistic model, we would see nuclei vibrating around their “fixed” location and the entire molecule rotating. And once in a while, a molecule will rearrange its structure, taking on an entirely different shape. That is, it becomes an isomer of that molecule. Answering a number of questions in chemistry and physics depends on understanding the rate of isomerization of molecules: how fast or slowly they change their shapes. Recently, Dr. Koushik Saha, Dr. Oded Heber and their colleagues in the group of Prof. Daniel Zajfman of the Weizmann Institute of Science’s Particle Physics and Astrophysics Department developed a way to time this rearrangement and discovered it can be much, much slower than any rates that had previously been reported for an isolated molecule.
Any change in the shape of a molecule – even reversing the structure to create a mirror image – drastically affects its properties. This starts with the vibrational rotation around its internal fixed positions and extends to its chemical properties. Thus understanding the process of isomerization is crucial to numerous fields of study. Heber explains that gauging how long it takes a molecule to change its shape -- assuming that all the conditions (enough energy, for example) for this change to occur exist – has been estimated on the basis of the molecules’ dynamics – for example, on the measurements of the speed of the atomic nuclei as they move through a single rotation. Typical rotation times of a molecule are of the order of one picosecond to hundreds of picoseconds, depending on the size of the molecule; other experiments with isomerization seemed to agree with these numbers.
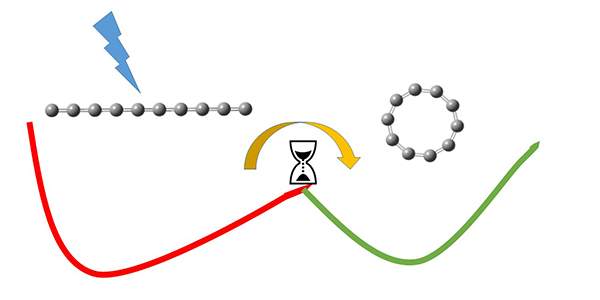
Heber and his colleagues managed to measure the isomerization time in a chain of 10 carbon atoms – plus one extra electron – as this chain transformed into a ring. To get the rearrangement under way, the researchers zapped it with a controlled laser pulse that excited the carbon molecule. Rather than picoseconds, the process of transformation continued for up to hundreds of microseconds. In other words, it was up to a million times slower than any other experiments had shown for isolated molecules. To explain this behavior, the scientists developed an improved statistical model.
The new model may help astrophysicists find answers to some open questions about complex molecules in outer space. These have been measured with spectroscopy, in conditions of very low density. Among those complex molecules found in outer space are amino acids – the building blocks of proteins – as well as other large hydrocarbon molecules, so the model may help researchers understand the chemistry of these biological molecules in the cosmos.