Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

The Weizmann Institute of Science’s Flow Cytometry Unit, says Dr. Ziv Porat, is busy day and night, weekends and holidays. Porat, in addition to overseeing the use of the flow cytometers, is in charge of the Imaging Flow Cytometry equipment: “In the four years that the instrument has been in our lab, imaging flow cytometry has played a role in over 30 published papers, many of them high-profile; there have been over 150 research projects that have used it, and we still have not realized the full potential of this instrument.” The Weizmann Institute’s Flow Cytometry Unit is headed by Dr. Ayala Sharp and Eitan Ariel.
In conventional flow cytometry, Porat explains, living or fixed cells placed in suspension pass through the device one by one while lasers detect the quantities of various markers – proteins, DNA or other molecules labeled to glow in a number of fluorescent colors. This enables the accurate quantification of up to 18 different markers for each cell, as well as the sorting of different cell populations. Imaging flow cytometry combines the principles of this method with microscopy, in effect adding visuals: a microscope camera enables the scientist to view individual cells, even as they flow through the instrument at a rate of up to 5000 a second. Such imaging reveals, for example, the shape and structure of the cell and the locations (or colocalization) of specific proteins, and it enables the analysis of cellular processes or captures DNA in various stages of cell division.
When scientists come to him, says Porat, he helps them to design their experiments with the imaging flow cytometry instrument and to read and analyze their results afterward.
For example, Prof. Nir Friedman of the Weizmann Institute’s Immunology Department had a question: Why do many cells have multiple versions of genes that do the same thing? Using imaging flow cytometry combined with live-cell imaging, they were able to figure out what happens when an immune cell employs two different genes to initiate an immune response. They focused on NFAT proteins – which respond to a stimulus by entering the nucleus and binding to the DNA, thus activating further genes, and are actually produced in five different versions within the cell body.
By labeling two of these versions – NFAT1 and NFAT4 – the researchers were able to observe how each protein entered the cell nucleus: up to an hour after the signal. Imaging Flow Cytometry, something like a highly precise traffic report, showed the shuttling of these proteins in and out of the nucleus in tens of thousands of cells. This report revealed that each version worked differently: NFAT1’s entrance and exit were slow and smooth, in contrast with NFAT4’s short, jumpy moves.
This suggested to the researchers that even though the two versions of NFAT activate a shared set of genes, their different dynamics may enable cells to produce a wide range of responses to a signal, based on its temporal patterns.
Porat began his relationship with the Weizmann Institute with MSc studies in Prof. Michal Schwartz’s neurobiology group, after which he conducted his doctoral research in Prof. Chaim Kahana’s molecular genetics group, followed by postdoctoral research in Prof. Benjamin Geiger’s molecular genetics group. It was the breadth of his experience in life sciences research that prepared him for working with instruments that are used by many diverse research groups. He joined the Biological Services Department in 2011, along with the ImageStreamX Imaging Flow Cytometry instrument.
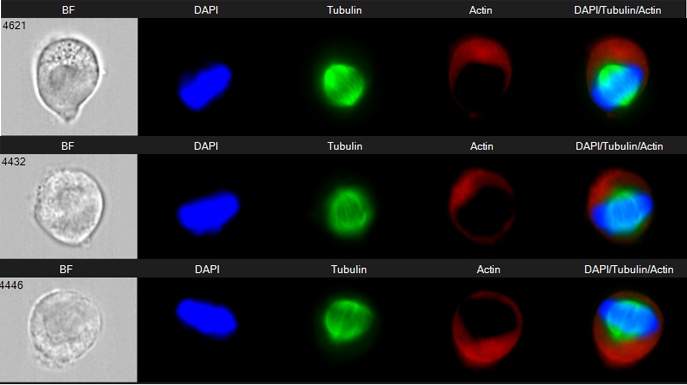
The many and varied research projects Porat has been involved in include the following: