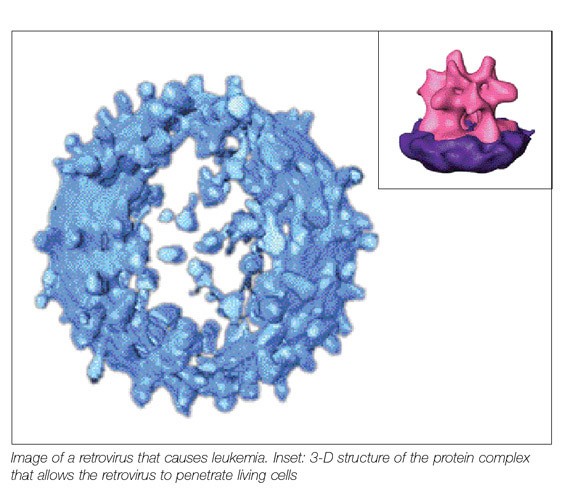
Retroviruses are the ultimate sneak thieves of the microscopic world. The outer envelopes of these viruses, some of which cause AIDS or cancers such as leukemia, are spiked with protein assemblies that are specialized tools for breaking and entering. There’s no need to force windows or pick locks: The retrovirus' surface proteins simply cause the membrane of the virus to fuse with the cell's outer membrane. Once the two are fused, the genetic material at the heart of the retrovirus, RNA, makes itself at home in the cell, stealing the cell’s most basic equipment to make copies of itself.
Viral surface proteins, in turn, make attractive targets for drugs and vaccines – blocking them might stop infection before it can take place. Like detectives on the path of a criminal, scientists need information – mug shots, fingerprints, eyewitness reports – to help them capture their target. A team of scientists at the Weizmann Institute of Science and the Max Planck Institute for Biochemistry has now obtained a close-up, 3-D portrait of a large protein complex responsible for retroviral breaking and entering. Results of their work appeared in the Proceedings of the National Academy of Sciences, USA.
The retrovirus protein complex studied by the group recognizes and binds to specific sites on the cellular membrane and mediates the fusion process at the very onset of infection. However, the shape of this complex and the way it works had long evaded efforts at detection by various scientific groups. The difficulty was that crystallization, the leading method of preparing proteins for structure solving, does not work well with the elaborate, envelope-bound complexes, which tend to fall apart when removed from the virus membrane. Dr. Deborah Fass of the Weizmann Institute’s Structural Biology Department had managed to determine the structures of assorted parts of the complex in the past, but she needed a better understanding of how the complex works as a whole
.
To accomplish her goal, Fass and student Nathan Zauberman teamed up with scientists from Max Planck’s Molecular Structural Biology Department in Martinsried, Germany, to try an alternative method of getting an image of the complex. They turned to the electron microscope, a standard tool for observing larger structures such as cell sections. Viewing a single, relatively small protein complex pushed the limits of this technology, but the Max Planck group, expert at developing both the hardware and the software required for visualizing biological structures with electron microscopy, proved up to the task. The technique they used, known as cryo-electron tomography, involves quick-freezing the viruses in liquid ethane, capturing snapshots of them at various angles and then combining the snapshots to create three-dimensional pictures. From dozens of these digitized 3-D pictures of whole viruses, hundreds of protruding surface protein complexes could be cut out, aligned and averaged. Though the resulting image did not have quite as high a resolution as images obtained through crystallography, it allowed the scientists to get a complete and fairly detailed picture of this important protein complex all in one piece and in its natural setting. “After years of trying to imagine how the pieces fit together, suddenly we had the actual structure right in front of us. Some aspects of it looked familiar, but others were completely unanticipated,” says Fass.
The scientists were surprised to note that the shape of the complexes on retroviruses bore little resemblance to other known viral envelope protein structures such as those on flu viruses. They also saw strong evidence that the protein complex undergoes a radical change in the shape and arrangement of its component parts as it attaches to cells and initiates membrane fusion. Fass was able to see how a smaller protein piece she had previously isolated and analyzed by crystallization fit into the whole, giving her further clues as to how the virus locks onto the cell membrane.
The retrovirus used by Fass and the team is similar to that which causes leukemia in humans. They hope, with further research, to come to understand the conformational changes the envelope protein complex undergoes as it works, and to find ways to stop those changes from taking place, thus disabling a sneak thief’s main tool for breaking into cells.
Dr. Debora Fass’s research is supported by the Clore Center for Biological Physics; the Helen and Milton A. Kimmelman Center for Biomolecular Structure and Assembly; and the Leukemia Research Foundation. Dr. Fass is the incumbent of the Lilian and George Lyttle Career Development Chair.
Reverse reproduction
The genetic material of retro-viruses such as HIV and the virus that causes leukemia is found in single strands of RNA, rather than in the double-stranded DNA that all living things employ to store genetic information. Unlike living cells, viruses cannot reproduce on their own, and thus they must “hijack” the machinery of a living cell to do so. For this purpose, the retrovirus carries with it a special enzyme, called reverse transcriptase. In normal transcription, RNA is formed from a DNA template, but reverse transcriptase turns this process around, lining up a DNA sequence to match the “letters” in the viral RNA. The “viral” DNA inserts itself into the host chromosome, where it remains to manufacture more retroviruses.