Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
There is enough energy striking the sea every day, in the form of sunlight, to bring it to a boil. Luckily, water molecules have unique properties that limit the absorption of heat from visible light, so the enormous bulk of water in the seas, lakes and oceans stays cool enough to sustain life. Researchers at the Weizmann Institute of Science have exploited these wondrous properties of water in unique experiments with freezing that enabled them to control the process of crystallization from its inception, creating ice crystals all oriented in the same direction.
The ability to control where crystals form, as well as their size and orientation, could be a great research tool for learning more about crystallization. And controlling the structural features of crystals, in particular their orientation, is important for obtaining desired properties in various materials used in electro-optical instruments, medical devices and a host of industrial applications.

Prof. Leslie Leiserowitz and Dr. Iftach Nevo of Weizmann’s Materials and Interfaces Department, together with colleagues, designed experiments aimed at achieving controlled freezing of so-called supercooled water in the laboratory of Prof. Dan Oron in the Physics of Complex Systems Department. Supercooled water normally remains liquid at temperatures well below zero degrees Celsius provided it’s sufficiently pure – that is, lacking foreign particles that may host the formation of ice nuclei. The scientists used laser light to coax it into homogeneous crystallization without the need for foreign particles – and much more readily.
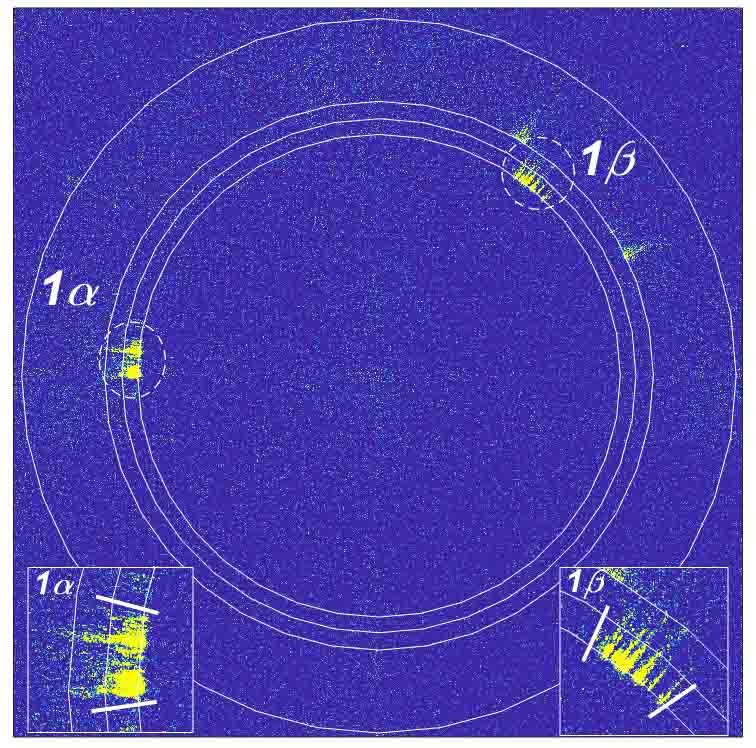
They built an installation that consisted of a cooling chamber in which the water was irradiated with two vertical laser beams aligned with one another. The researchers used green laser light, which is hardly absorbed by the water, and illuminated the water drops in a series of pairs of short laser pulses. A video of the experiment showed that the water began to crystallize under the effect of the laser pulses at a temperature some ten degrees higher than without the laser illumination.
The dual laser pulse in our experiment gave the molecules more time to turn the ‘high five’ into a ‘handshake'
Next, the Weizmann scientists formed a collaboration with researchers from the European Synchrotron in Grenoble, France, repeating the experiments in the synchrotron. This time, 100 nanoseconds after being illuminated by the two pulsed laser beams, the water drop was irradiated with a pulsed X-ray beam. The three beams were perpendicular to one other and met in the center of the drop. The diffraction patterns of the X-rays revealed the details of the crystallization at the atomic level. They confirmed that the ice crystals began to form homogeneously at the collision point of the laser beams and showed that the crystals at that point were basically oriented in the same direction – a direction that differed from the one in which ice nucleates naturally. Rather, it was parallel to the direction of the electric fields generated by the laser beams.
Based on physical models, the researchers propose an explanation for these findings. They suggest that the water exposed to the laser light absorbed little or no heat from the beam, but it was greatly affected by the beam’s electric field. Isolated water molecules are naturally polar, and their hydrogen bonds, which bring together hydrogen and oxygen atoms of neighboring molecules, are easily polarized even further. The extra polarization caused those adjacent molecules that had hydrogen bonds already oriented in parallel with the laser’s electric field prior to the illumination to experience a greater attraction to one another. This attraction lasted a relatively long time: throughout the prolonged exposure to the dual pulse. In this manner, the laser beams promoted the formation of hydrogen-bonded ice crystals aligned with the laser’s electric field.
Iftach Nevo explains: “Ice nuclei begin to form all the time in supercooled water, but they quickly disintegrate before getting a chance to connect – like two kids that run around, high fiving one another without joining hands – so the water stays liquid. The dual laser pulse in our experiment gave the molecules more time to turn the ‘high five’ into a ‘handshake,’ and this is what helped the crystals grow."
The experimental design used in the study may now be applied to control the crystallization of materials other than water – for continuing basic studies of the crystallization process or with a view to potential industrial applications. The study has been selected by the Journal of Chemical Physics for its 2020 Editors’ Choice collection of that year's most innovative and influential articles.
Study participants included Dr. Sabrina Jahn of the Materials and Interfaces Department; Dr. Nir Naftali; Dr. Yishay Feldman of the Chemical Research Support Department; and Dr. Norman Kretzschmar, Dr. Matteo Levantino and Dr. Michael Wulff of the European Synchrotron.
