Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
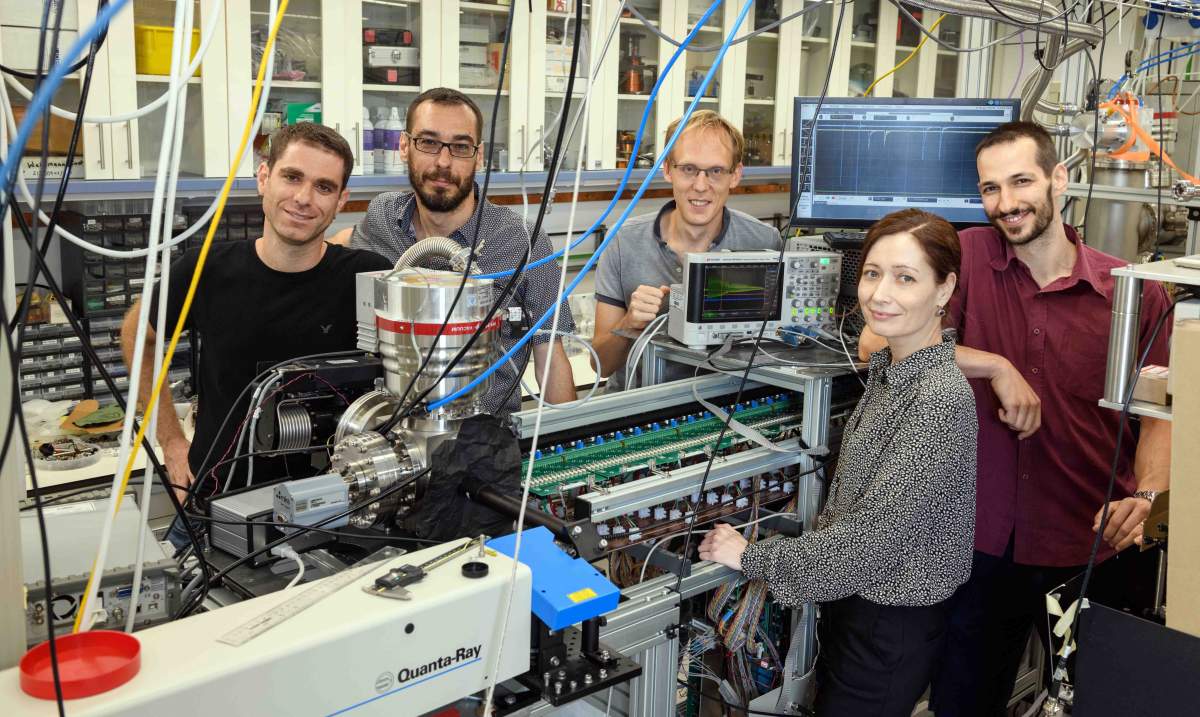
In the hot summer of 2019, everyone’s had a hard time cooling down. This is especially true for certain simple molecules: Scientists would like to cool them down to close to absolute zero, the lowest temperature possible – approximately -273oC. Yet this goal is continually sought because near this extreme, the fundamental nature of matter is revealed, and the properties of molecules can be studied with unparalleled precision.
Prof. Ed Narevicius of Weizmann Institute’s Chemical and Biological Physics Department explains that to cool, one must understand heat, which is nothing more than the constant, random motion of atoms or molecules. Scientists has long ago managed to cool atoms down to fractions of degrees above absolute zero using “boxes” made of laser beams coming from different directions. In effect, the lasers trap the atoms and significantly slow their natural motion. But this method, which has proved to be efficient for cooling atoms, cannot, for various reasons, be used on molecules.

Narevicius and his group decided to take a different approach to cooling molecules – that of evaporation. Evaporation of rubbing alcohol or aftershave produces a cooling effect on the skin because the molecules of alcohol take some of the skin’s heat with them as they move off into the air. And hot soup cools down by letting the hottest water molecules evaporate. Similarly, a “soup” of cold molecules can be cooled down further by continually releasing the hottest ones.
But in order to cool such a “soup” of molecules, the group realized they would first need a sort of “bowl” to contain them – not a trivial task since no container of glass or stainless steel can be cooled to such extremely low temperatures. The system they ended up building was technologically quite complex.
A “soup” of cold molecules can be cooled down further by continually releasing the hottest ones
The scientists, including research students Yair Segev and Michael Karpov, postdoctoral fellow Dr. Martin Pitzer, staff scientist Dr. Nitzan Akerman and Julia Narevicius, first introduced oxygen gas into a vacuum chamber at room temperature. Like all gases, it naturally expanded into the container – a widening beam of molecules spreading out into the empty space. When a gas expands, it cools; in the experimental conditions in the lab, the temperature of the gas was measured at around one Kelvin (that is, one degree above absolute zero).
But the molecules were still moving. In fact, they were flying through the vacuum chamber at the reckless speed of around 400 meters a second, making it extremely hard to catch them. In order to do so, the team used strong magnetic fields: The oxygen molecules in this system behaved like small magnets, and thus could be manipulated with the magnetic fields.
To achieve these fields, the group used special superconducting wires that can carry high electrical currents for unlimited duration without heating up; similar wires can be found in MRI machines. In the system they developed, the researchers could quickly trap a large number of molecules in a confined space – a situation that significantly increases the rate of collisions between the molecules. These collisions are needed to transfer heat between molecules, some heating up and evaporating out of the trap while others cool down. The group was able to identify many such collisions occurring between the molecules inside the magnetic trap.
The experiment was the first successful attempt to catch cold molecules without using lasers and which showed conclusive evidence of collisions. Now, Narevicius and his group can use their system to further push molecules closer to absolute zero, into the realm where quantum mechanics is expected to change the very nature of their behavior. The results of this research were recently publisahed in Nature.
Prof. Edvardas Narevicius' research is supported by the Helen and Martin Kimmel Award for Innovative Investigation.