Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Imagine trying to build a Colosseum-type edifice – including arches, vaults and various protrusions – while abiding by two strict rules: Only one type of brick may be used, and these bricks are required to be placed precisely, one against another, in a symmetrical arrangement. Not even a bit of mismatching is allowed. At best, you would be able to erect a chambered high-rise tower. Nature has similar laws for the construction of single crystals.
The rules for the formation of single, molecular crystals are so strict – they must be sharp-edged, continuous, single-compartment structures – that it is inconceivable to imagine these principles being broken. Until now, that is. Weizmann Institute of Science researchers have managed to create structures that are a complete paradox: single, continuous crystals that have multiple domains, an asymmetric shape and curved lines; they are as complex as one could expect from a “monumental” structure. This unique class of organic materials was recently reported in Nature Communications. Because crystal structure plays a critical role in determining the properties of a material, the Weizmann scientists plan to further investigate these new structures and to apply their special assembly approach to different types of “molecular bricks” that may help create highly-versatile crystalline materials.
There are many open issues in to crystal engineering, although some date back to the work of Louis Pasteur in the 19th century – for example, how to control the growth of crystals so they will have a uniform size and shape, or how to control chirality. Chiral molecules are identical in their makeup but come in two mirror-image forms that, like hands, appear in “left” or “right” versions that cannot be superimposed. Chiral crystal formations can be helical – spiraling in either clockwise or anticlockwise directions, depending on the molecules’ “handedness.”
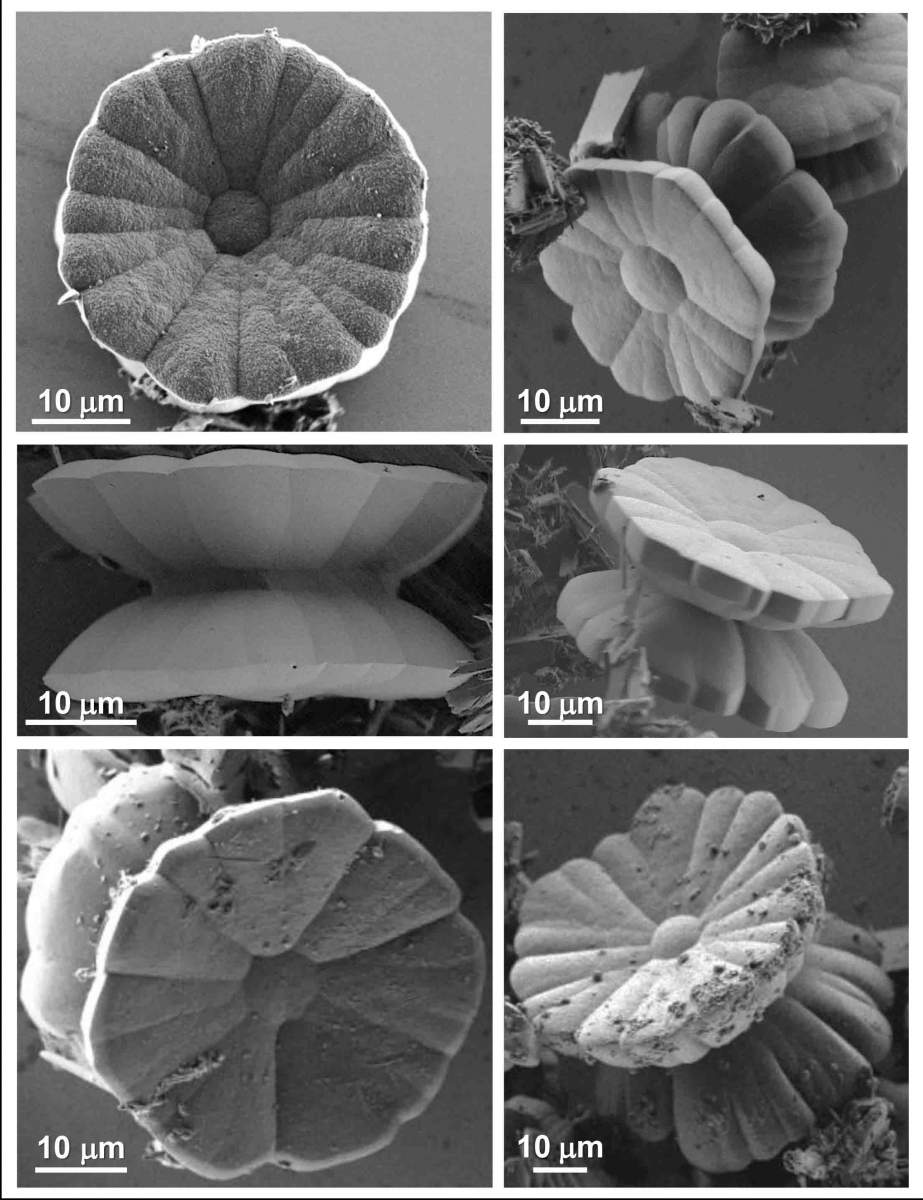
Weizmann Institute scientists, spearheaded by Italian postdoctoral student Dr. Maria Chiara di Gregorio, Senior Staff Scientist Dr. Michal Lahav and Prof. Milko van der Boom, all of the Organic Chemistry Department, have touched upon these questions. Over the years, they have perfected their method of creating single crystals that are highly complex in appearance, and they have now added a twist – literally. The crystal structures they created are shaped like a yo-yo, with the two halves spiraling in opposite directions; on top of this structure, the appearance of the two disks themselves is flower-like, with numerous chiral “petals” growing around a “stigma” at the center. “This is surprising, since the ‘building blocks’ are all symmetrical, non-chiral molecules,” says di Gregorio. “The petal-like appearance suggests that these ‘should be’ polycrystalline structures – that is, possessing multiple ‘chambers’ – rather than single crystals.”
To understand how this petalled, chiral structure arose from non-chiral molecules, the scientists used several techniques to examine the crystals at three different levels: the morphological (3-D shape) level, the molecular level, and then at a level somewhere in between – the electron density distribution.

Using scanning electron microscopy, they were able to construe four stages of crystal growth that could be defined on the morphological level. “Baking” organic molecules together with metal atoms in solution at the right temperature yields shapeless, non-chiral cylindrical structures. These are the “flower buds" that, in the following stages, transform into chiral objects. First they develop into two twisted hexagonal structures, and the petals then begin to grow and arrange themselves asymmetrically on the top surface of the two hexagons in a propeller-like fashion, taking on a clockwise or anticlockwise direction. In the final stage, the crystals develop into the well-defined yo-yo structure, with its multiple domains giving it aflower-like appearance.
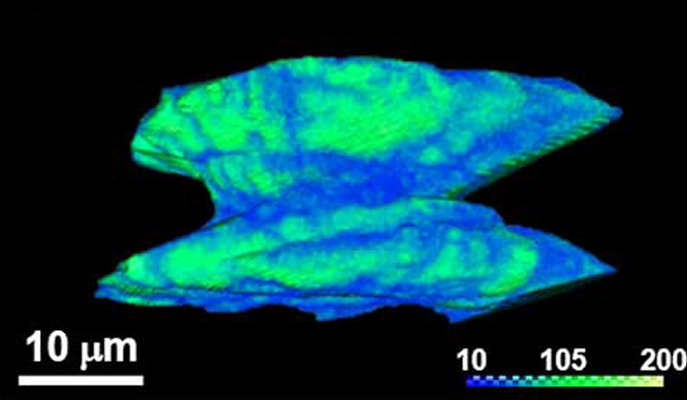
Investigating the structure further using micro-computed tomography (micro-CT) – an unconventional method in the 3D analysis of metal-organic crystals – the scientists revealed hidden details from the “in between” electron density distribution level. Indeed, the measurements reveal a continuous single spiral motif spanning the entire structure from base-to-base, suggesting that despite the complex shape, it is a single chiral crystal.
At the molecular level, X-rays applied by Dr. Linda Shimon of the Department of Chemical Research Support, showed the crystal structure clearly and provided conclusive evidence for the single crystal makeup of the complex yo-yos. The X-ray patterns also revealed “spiral stairways” – porous, chiral channels that extend across the entire structure from top to bottom.
These twisted crystals were so contrary to nature that the researchers had the structure independently confirmed by a crystallographer in New York.
“These results are exciting on a fundamental level, as we have managed to create a completely unique class of material,” says van der Boom. The findings might find applications, he adds, in the design of new porous materials, for example for the storage of environmentally-friendly fuels such as hydrogen, or for capturing carbon dioxide from the atmosphere. And they could also be used for improving catalysis in various chemical processes.
Also participating in the research were Drs. Vlad Brumfeld and Lothar Houben of the Chemical Research Support Department.
Prof. Milko van der Boom's research is supported by the Ben B. and Joyce E. Eisenberg Foundation; the Richard F. Goodman Yale/Weizmann Exchange Program; and the estate of Emile Mimran. Prof. van der Boom is the incumbent of the Bruce A. Pearlman Professorial Chair in Synthetic Organic Chemistry.