Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Dr. Amnon Bar-Shir says the idea came to him from a picture in a book: a photo of a purple crystal known as fluorite (calcium fluoride). Bar-Shir is a magnetic resonance imaging – MRI – investigator in the Weizmann Institute of Science’s Organic Chemistry Department. The rock’s beautiful color first attracted his attention, but he also noted it contains the element fluorine, which had been touted for many years as a possible tracer for MRI – one that would, theoretically, be able to image additional molecules over and above conventional MRI. Despite the potential that exists for fluorine-containing materials in MRI applications, the idea of using calcium fluoride was not, at first glance, promising: Its solid, crystalline structure should have kept it from showing up in MRI, which picks up signals from soft tissue, fluids or small soluble molecules.
Indeed, clinical MRI mostly images the water molecules in our bodies. More specifically, it images the hydrogen atoms in water as their nuclear spins align with the strong magnet in the equipment. Fluorine atoms return a nuclear magnetic signal that is very similar to that of water’s hydrogen, but they do so in a different frequency, meaning that if a tracer containing this element was to be developed, it could be used with existing methods and equipment. As opposed to water, fluorine is not naturally present in the body’s soft tissue. So injecting a safe, fluorine-based tracer into just the desired organ or tissue might provide a high quality image without all of the water’s background signal. But to create a signal in an MRI scanner, the tracer material must be designed to be labile (fluid or a soluble material). In Bar-Shir’s words, it somehow has to “freely tumble in solution,” that is, without the severely restricted mobility of a normal solid.
Bar-Shir: “I had this crazy idea that nanocrystals of calcium fluoride could somehow be made small enough to tumble very fast inside the body and thus have the characteristic mobility needed to produce their own unique MRI signal.”
At first Bar-Shir had put his idea on the back burner as he began pursuing several other, more prospective, avenues of MRI research. That changed the day that Dr. Idan Ashur came to his lab and asked to join his group. “Ashur had never worked with MRI, nor with biological imaging,” says Bar-Shir. “But he had lot of experience in one particular area that is relevant to our scientific efforts – making nanoparticles. We agreed he would try out my ‘impossible’ idea – magnetic resonance imaging with calcium fluoride nanocrystals. If it didn’t work, he would be free to move on to another lab.”
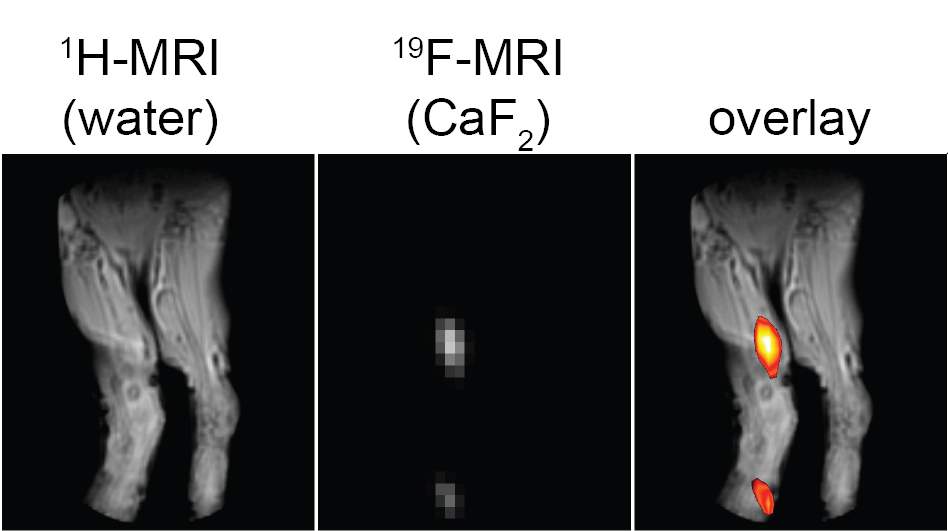
But Ashur, working as a senior intern in Bar-Shir’s lab, succeeded in producing tiny calcium fluoride nanocrystals, and the results were stunning: The nanocrystals he placed in solution in a test tube returned high MRI signals. Just four or five nanometers across, the tiny crystals were apparently small enough to tumble in the solution and create a signal.
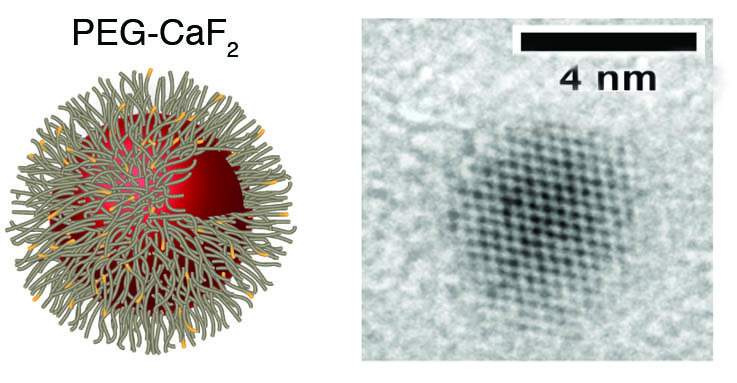
Next, with postdoctoral fellow Dr. Hyla Allouche-Arnon, the researchers decided to adapt the nanocrystals for biological imagining. They turned to a technology used in the pharmaceutical industry for extending the circulation lifetime of small drug molecules or injectable nanoparticles. Called PEGylation, it involves coating nanoparticles in a biocompatible substance called polyethylene glycol, which increases their stability in biological media.
Creating coated nanocrystals turned out to be a complex process, but one that paid off. After the scientists injected the new nanocrystals into mice, they were able to obtain clear images of extensive inflammation processes in the lymph nodes. Further investigation showed that the nanocrystals were taken up by cells known as macrophages – the body’s “clean-up” crew – and removed to the lymph nodes. When there were large numbers of macrophages in the bloodstream due to inflammation, the lymph nodes were strongly highlighted in the MRI images.
“We started with an impossible idea and took it all the way to demonstrating its effectiveness in living tissue.”
In continued experiments, the research team showed that other fluorine-based compounds might also be used in nanocrystal form, possibly in combination with one another. They created nanocrystals of strontium fluoride and found that its MRI signal is different from that of the calcium fluoride. The team managed to display multicolored images using the two types of nanocrystals, suggesting the technique might be used in the future to monitor multiple targets simultaneously.
The results of these experiments were recently published in Angewandte Chemie.

Markers explicitly designed to image the early stages of inflammation clearly might be the basis of a powerful diagnostic tool for various pathologies including the onset and progression of such diseases as Alzheimer’s, Parkinson’s, multiple sclerosis and even cancer. And additional molecules – for example, antibodies that target specific cell types – could theoretically be attached to the PEG-coated nanocrystals, further expanding the capabilities of MRI. The nanocrystals on their own could also open new avenues of imaging in material science, as they have properties of both solid crystals and small, flowing molecules.
“As far as I know, this is the first time magnetic resonance signals have been obtained from elements within nanocrystals,” says Bar-Shir. “We started with an impossible idea and took it all the way to demonstrating its effectiveness in living tissue.”
Yeda Research and Development, the technology transfer arm of the Weizmann Institute of Science, has filed a patent application and is working with Bar-Shir’s lab to develop advanced versions of the calcium fluoride tracers for commercial use.
Dr. Amnon Bar-Shir’s research is supported by the Moross Integrated Cancer Center; the Helen and Martin Kimmel Center for Molecular Design; the Ilse Katz Institute for Material Sciences and Magnetic Resonance Research; and the Lord Sieff of Brimpton Memorial Fund. Dr. Bar-Shir is the incumbent of the Helen and Milton A. Kimmelman Career Development Chair.