Better known for their destructive feeding habits, cabbage looper moth (Trichoplusia ni) caterpillars are proving to be a useful new tool in the production of human protein.
The ability to produce large quantities of specific human proteins is crucial for life sciences research, and it is at the heart of the biotechnology and biomedical industries. The most traditional and widely used protein expression methods are based on cell cultures: Human DNA is introduced into bacteria, yeast or animal cell lines, where it “hijacks” the cells’ protein-making machinery and tricks it into producing human protein instead.
Though effective, these methods can be very expensive and time-consuming. A new protein expression system using whole insect larvae offers a potential alternative, cost-effective method of producing large quantities of human proteins. Before such methods can be employed, however, one question must be answered: Are the proteins generated comparable in structure and function to those produced by the traditional techniques?
To find out, Drs. Tamara Otto and Douglas Cerasoli of the US Army Medical Research Institute of Chemical Defense and Dr. George Buchman of Chesapeake PERL Inc. turned to Dr. Harry Greenblatt and
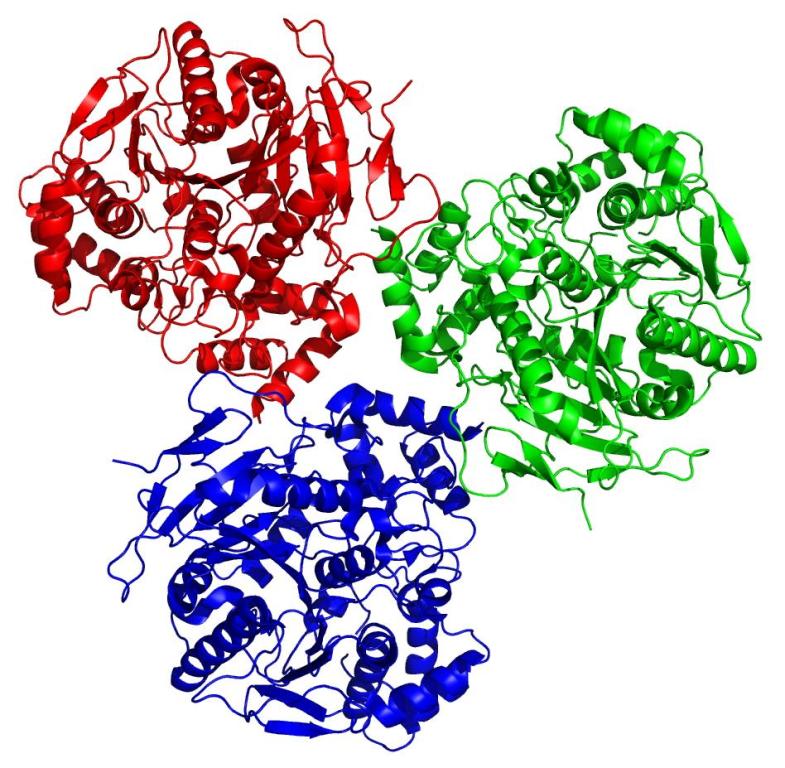
Prof. Joel L. Sussman of the Weizmann Institute’s Structural Biology Department (Faculty of Chemistry) to decipher the structure of the enzyme human carboxylesterase 1, produced using the new system. This enzyme, already well characterized using cell culture techniques, is expressed primarily in the liver and is thought to be responsible for the breakdown of drugs. With slight modifications to its design, it also holds promise as an antidote against insecticides and even nerve gas agents.
Using the state-of-the-art facilities at the I
srael Structural Proteomics Center (ISPC) at the Weizmann Institute, of which Sussman is Director, and combining various functional analysis, crystallization and data collection methods, Greenblatt was able to crystallize the human carboxylesterase 1 enzyme isolated from the larvae and confirm that its structure – and function – was identical to previous examples of this enzyme isolated from cultured insect cells.
This proof of principle study validates the use of whole insects as a new resource for producing human proteins. “Not only are these moth larvae easy to grow and manipulate,” says Greenblatt, “they are also cheaper than cell culture techniques and even produce a larger quantity of protein.”
Sussman: “So far, we have tested only the human carboxylesterase 1 enzyme; but if this new system proves to work for other proteins as well, it will provide an invaluable tool for researchers and industry, alike.”
Prof. Joel Sussman’s research is supported by Yossie and Dana Hollander, Israel, the Samuel Aba and Sisel Klurman Foundation; the Bruce H. and Rosalie N. Rosen Family Foundation; and the Nalvyco Trust. Prof. Joel Sussman is the incumbent of the Morton and Gladys Pickman Professorial Chair in Structural Biology.