Body joints are superbly lubricated. Wherever two bones meet in our body, joints allow us to grasp, bend over, run or dance - and they’re supposed to last a lifetime.
Mimicking key design elements of this biolubrication system, physicist Prof. Jacob Klein of the Materials and Interfaces Department has recently created a synthetic lubricant that cuts friction a thousand-fold or more. The study, published in Nature, could lead to a range of applications - from longer-lasting micro-machines to biomedical products.
Previous studies had suggested that biolubrication systems, such as those in joints and eyes, maycontain hyaluronan molecules that coat the rubbing surfaces, shielding them from mechanical damage. Hyaluronan was also known to be strongly attracted to water.
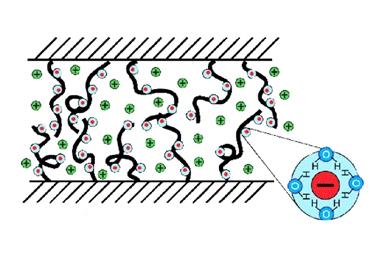
Klein and his colleagues suspected that in joints, hyaluronan may be attached to a thin cartilage layer covering the bone. Parts of the long, chain-like hyaluronan molecule stick out into the synovial fluid between the bones, resembling bristles on a brush.
The team developed a synthetic model that mimicked a double-brush system, anchoring two charged molecules (polyelectrolytes) to opposite-facing ceramic surfaces. The resulting system showed extremely effective friction resistance, particularly when exposed to a water-based solution. “The brushes strongly try to avoid each other, resisting contact even when an external force is applied to press them closer. This enables them to easily slide past one another,” says Klein.
The synthetic brushes were designed to imitate the electrically charged nature of biolubricants. The negative charge on the bristle tips then attracted water molecules - which in fact explains why the brushes performed most effectively in a water-based solution. “The water molecules are tightly bound by the charges, causing them to act like molecular ball bearings,” Klein explains.
Prof. Klein is the incumbent of the Hermann Mark Professorial Chair of Polymer Physics.