Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
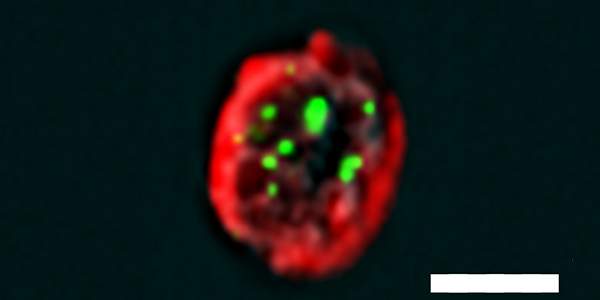
Advanced technologies have lately made such tremendous progress that we can extract genetic material from a single cell and learn practically all we want to know about a person’s genome. But the essential next step – studying the proteins encoded by the genome – often requires extracting proteins from millions of cells. Such large numbers, however, are unavailable for rare cell types in the body. In a study reported in Nature Communications, Dr. Jakub Abramson and his team in the Immunology Department of the Weizmann Institute of Science devised a method for studying proteins within a single cell isolated from the body of an animal or human. The researchers made use of rare cells, called mTECs, which function as genetic “selfies”: They create a nearly complete display of the organism’s genetic activity. Unlike other cells in the body, mTECs express – or activate – most of their genes.
The mTECs make up a tiny fraction of cells in our thymus – a small lymphoid organ that acts as the immune system’s headquarters – but without these “selfies” we would simply self-destruct. By expressing almost the entire protein-encoding genome, mTECs generate a “database” of all the body’s proteins that the immune system must learn to tolerate. Consequently, mTECs can kill or neutralize any developing T cells capable of reacting with the body’s own tissues, preventing them from reaching other tissues where they could ignite autoimmune “friendly fire.”

To study protein interactions within an mTEC, doctoral student Ayelet Avin, together with postdoctoral fellow Dr. Maayan Levy and Dr. Ziv Porat of the Life Sciences Core Facilities Department, developed a new method they called PLIC for proximity ligation imaging cytometry. PLIC works through a combination of two technologies: one that labels protein interactions or modifications with fluorescent probes, and one that can determine the location of numerous fluorescent labels at the same time, on the level of a single cell. The result is a highly sensitive method that makes it possible to visualize and quantify protein interactions or modifications within a single cell, even when the proteins are present in minute amounts.
The method enabled the scientists to reveal in great detail – and for the first time in the natural or physiological context of the organism – the mechanism by which mTECs prevent the immune system from mistakenly launching an autoimmune attack. They showed that an mTEC’s activity depends on an interaction between two key proteins: one encoded by a gene known as AIRE (AutoImmune REgulator) and another encoded by a regulator gene called Sirt1. They also found that Sirt1 activates AIRE by stripping it of a chemical tag known as an acetyl group. Moreover, the researchers managed to demonstrate experimentally that AIRE proteins bind to one another to form active complexes – an action that had been suggested by Abramson’s earlier work.
Without these “selfies” we would simply self-destruct
Abramson says these insights can add to our understanding of autoimmune disorders as they may tell scientists where to look for molecular defects. In particular, experimental evidence that Sirt1 is involved in preventing autoimmunity suggests that mutations or “variant spellings” in the Sirt1 gene may contribute to a number of autoimmune disorders. The research can also help explain the roles that mutations in the second gene, AIRE, play in autoimmunity.
“The ability to observe protein interactions on the level of a single cell provides major advantages because it enables the study of extremely rare cells in their natural form, the way they appear in the organism, without the distortions that inevitably occur when cells are grown for study in large numbers outside the body,” says Abramson.
In addition to the possible advances in autoimmune research, the Weizmann scientists showed that PLIC can be applied to cells other than mTECs. The method may, for example, prove useful for studying stem cells, rare cells in the inner lining of the gut, or other cell types that are present in the body in extremely small numbers.
Dr. Jakub Abramson's research is supported by the Wohl Biology Endowment Fund; the Rising Tide Foundation; the Abisch Frenkel Foundation for the Promotion of Life Sciences; the Sy Syms Foundation; and the Ruth and Samuel David Gameroff Family Foundation. Dr. Abramson is the incumbent of the Dr. Celia Zwillenberg-Fridman and Dr. Lutz Zwillenberg Career Development Chair.