Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Can a protein found in a mosquito lead to a better understanding of the workings of our own brains? Prof. Ofer Yizhar and his team in the Weizmann Institute of Science’s Neurobiology Department took a light-sensitive protein derived from mosquitos and used it to devise an improved method for investigating the messages that are passed from neuron to neuron in the brains of mice. This method, reported today in Neuron, could potentially help scientists solve age-old cerebral mysteries that could pave the way for new and improved therapies to treat neurological and psychiatric conditions.
Yizhar and his lab team develop so-called optogenetic methods – research techniques that allow them to “reverse engineer” the activity of specific brain circuits in order to better understand their function. Optogenetics uses proteins known as rhodopsins to control the activity of neurons in the mouse brain. Rhodopsins are light-sensing proteins – they are most known for their role in organs like the retina rather than in the dark inner reaches of the body. But the rhodopsins in the brains of Yizhar’s mice enable him to control the activity of specific neurons when he and his team shine a minuscule beam of light into the mouse’s brain. He is especially interested in communication between neurons: What signals are getting passed through the synapses, those gaps over which the brain’s signals move? “We can detect the presence of the various neurotransmitters, but different neurons ‘read’ those neurotransmitters differently,” he says. “Optogenetics enables us to not only see the ‘ink,’ but really to decipher the ‘message’.”
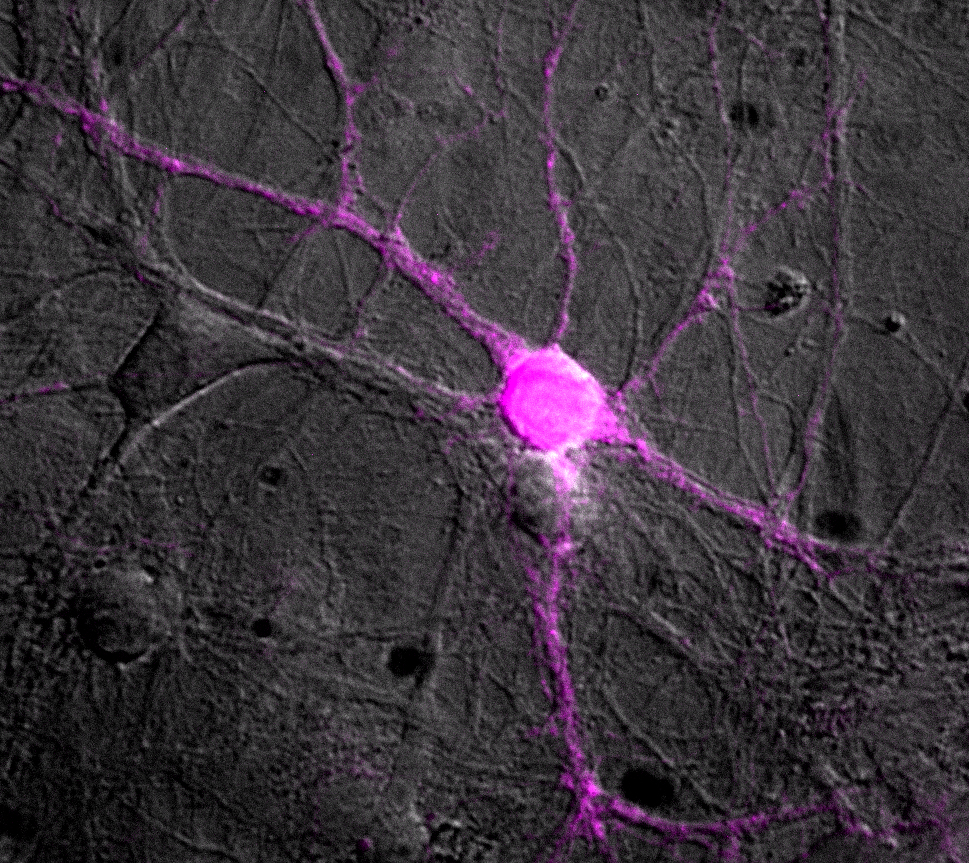
While optogenetic methods have produced a number of breakthrough results in labs around the world in recent years, they can be a bit finicky. In particular, the rhodopsins used for optogenetic studies tend to be imperfect when it comes to controlling the activity of synapses, the tiny junctions between neurons.
Rhodopsins are light-sensing proteins that are manipulated in optogenetic methods to reveal communication pathways in the brain
Yizhar and a large team of his trainees, including Dr. Mathias Mahn, Dr. Inbar Saraf Sinik and Pritish Patil, believed they could create a better version of the rhodopsins than those currently available. “We decided to look around and see what natural solutions exist out there,” says Yizhar. And nature, it turns out, contains a multitude of variations on the rhodopsin molecule – not only in animal eyes but also fish, insects, and even mammals carry them in various body parts; some possibly for regulating their circadian cycles, others for purposes as yet unknown. Thus, the team started out with a long list of potential rhodopsin proteins, and their first job involved assessing which ones were most likely to fill their experimental requirements, which primarily included light-gated proteins that are able to modulate synaptic activity. Eventually the researchers winnowed their list down to two – one taken from a pufferfish and one from a mosquito.
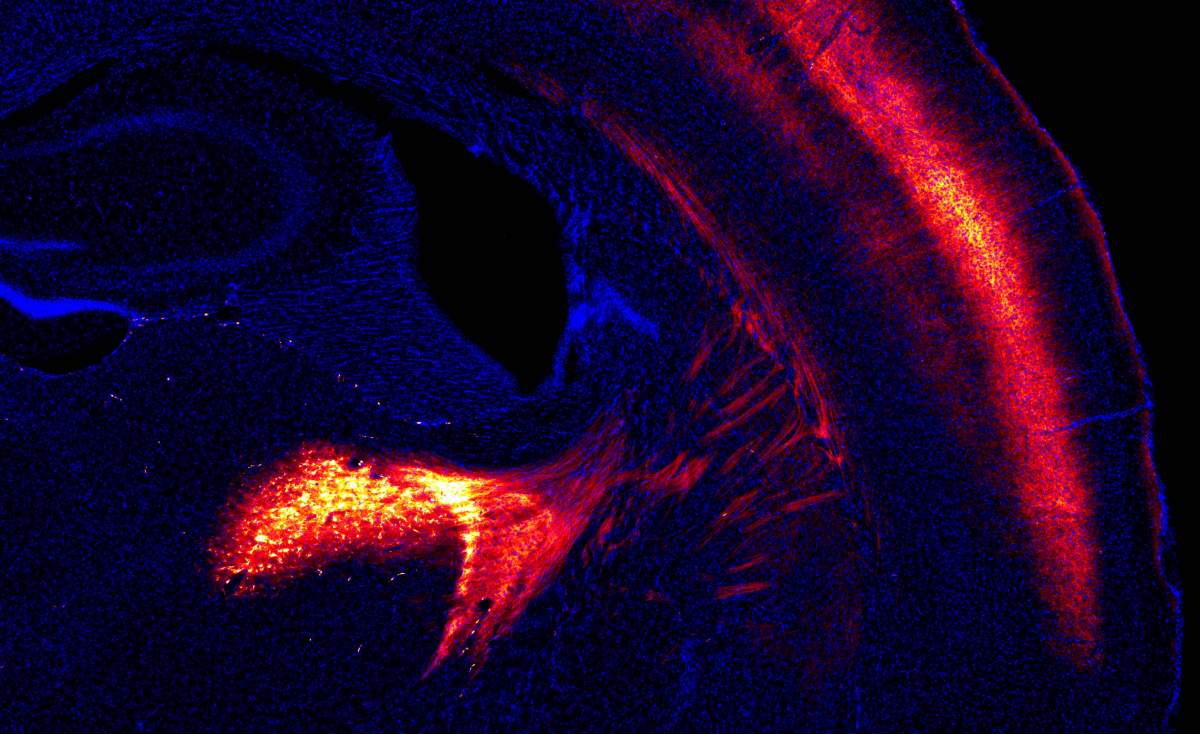
It was the mosquito rhodopsin that turned out to be the most suitable. To evaluate the efficacy of the new mosquito-derived tool, the researchers tested their method against a drug that is known to reduce the strength of the communication between neurons in the brain. They found that the interference was just as effective, and much more stable with the mosquito rhodopsin.
More than that: Unlike a conventional drug that affects numerous parts of the brain and is hard to control, the researchers found that since only neurons that produce the mosquito sensor are affected by the light, the modulatory effect on the brain’s synapses can be precisely controlled in both space and time – just by switching the light on or off in specific brain regions. They then validated the utility of the new tool by using it to block the release of the neurotransmitter dopamine on one side of the brain only: Illuminating the hemisphere expressing the mosquito rhodopsin with green light led to a one-sided bias in the behavior of these mice. In other words, they had created a tool that was precise, selective, and controllable.
“One of the major advantages of the mosquito rhodopsin is that it’s bistable – that is, it does not need refreshing – and it is potentially very specific, so that we can control just the precise synapses in which we are interested,” says Yizhar. “This is a very exciting technology, since it will allow us to discover the roles of specific pathways in the brain in a way that was not possible before. We think this mosquito protein could open the way to developing a whole family of new optogenetic tools for use in neuroscience research.” These scientific endeavors will receive a great boost within the framework of the new Institute for Brain and Neural Sciences – Weizmann Institute’s flagship project that is expected to bring together leading research groups from various fields, which will join efforts to unfold the mysteries of the brain.

At the beginning of 2021, Yizhar’s optogenetic research was included in Nature’s list of “Seven technologies to watch in 2021.”
Study authors also included Dr. Jonas Wietek, Dr. Julien Dine, Rivka Levy, Anna Litvin, Ido Davidi and graduate students Eyal Bitton, Shaked Palgi and Asaf Gat from Prof. Ofer Yizhar’s group – who worked alongside their European collaborators Dr. Simon Wiegert from the Center for Molecular Neurobiology in Hamburg, Dr. Benjamin Rost and Dr. Dietmar Schmitz at the Charite Research Hospital in Berlin, and Dr. Andreas Lüthi from the Friedrich Miescher Institute for Biomedical Research (FMI) in Basel.
Prof. Ofer Yizhar is the incumbent of the Joseph and Wolf Lebovic Charitable Foundation Chair for Research in Neuroscience.
Prof. Yizhar’s research is supported by the Ilse Katz Institute for Material Sciences and Magnetic Resonance Research; and the Adelis Brain Research Award.