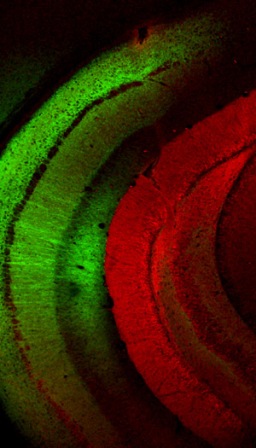
The idea of controlling the activities of individual brain cells originated with Francis Crick, one of the discoverers of the DNA double helix. Crick, who later in his life was involved in neurobiology research, predicted in the 1970s that scientists would find ways to actively manipulate brain cells, and even guessed it would be done with light. People have attempted to do this in various ways over the years, but it took the discovery, in 2001, of a light-sensitive protein in a microscopic alga to kick-start the field of optogenetics.
This protein is a member of a large family called rhodopsins, all of them built to absorb light. The algal rhodopsin, which helps the microorganism steer toward light, is unique in the way it works: Light prompts it to open channels in the cell membrane, letting various charged ions in or out and thus changing the cell’s internal chemistry. Since neurons fire off charged signals to one another through similar channels, scientists thought that these rhodopsins might finally give them the control they sought. Surprisingly, the algal rhodopsins functioned quite well in sophisticated mammalian nerve cells.
The first report on a marriage between an algal protein and a nerve cell was published in 2005, and it was this seminal paper that set Yizhar on his path. As he neared the end of his doctoral research at Tel Aviv University and was contemplating postdoctoral positions, he was searching for ideas in the pages of neuroscience journals. “I wanted a subject that would excite me,” he says. That seminal paper was the spark he was looking for, and Yizhar set off to do his postdoctoral research in the optogenetics lab of the paper’s lead author, Dr. Karl Deisseroth, at Stanford University.
There, Yizhar joined a group of young researchers in developing the toolkit for the nascent field. Beginning with nerve cells grown in culture and progressing to genetically engineered mice in which selected brain cells were activated by light pulses from tiny implanted optic fibers, the team continued to demonstrate the potential of the method. By now, says Yizhar, the toolkit has advanced to the point where different neurons can be made to respond to different colors of light, enabling scientists to work with more than one cell type at a time. The developers have made the toolkit available to other researchers and, so far, over a thousand labs worldwide have requested it.
In his Weizmann lab Yizhar intends, among other things, to continue research he began at Stanford in an area of the brain called the prefrontal cortex. This is where such higher functions as goal-directed behavior and working memory take place; faulty circuitry in this area is implicated in a number of psychiatric problems. Yizhar and the Stanford team tested a theory that both autism and schizophrenia might be tied to an imbalance in the activities of two types of neurons controlling these circuits. Indeed, when the researchers used their light-activated optogenetic tools to create such an imbalance in lab mouse brains, they saw behavior associated with autism.
Yizhar emphasizes that we will not be curing psychiatric disorders anytime in the near future with implanted optic cables. Rather, optogenetics will give researchers powerful tools that should enable them to pinpoint the sources of malfunctions and hopefully lead to the design of effective treatments.
Back to Rehovot
Dr. Ofer Yizhar grew up in Mazkeret Batya, near Rehovot, and attended the Israeli Arts and Science Academy high school in Jerusalem. He received his B.Sc. from the Hebrew University of Jerusalem, and his M.Sc. and Ph.D., in neurobiology, from Tel Aviv University.
He lives on campus with his wife Lital, a breast-feeding counselor, and their three children. In his spare time, Yizhar enjoys swimming, rock climbing and music.
Dr. Ofer Yizhar's research is supported by the Adelis Foundation; the Candice Appleton Family Trust; and the Clore Center for Biological Physics. Dr. Yizhar is the incumbent of the Gertrude and Philip Nollman Career Development Chair.