Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
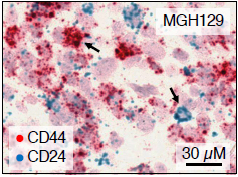
Glioblastoma, a typically incurable brain cancer, is a master of diversity. Not only do the tumors differ from one patient to the next, but cells within each tumor differ greatly from one another. In a study published recently in Cell, researchers at the Weizmann Institute of Science, working in collaboration with Boston researchers and physicians, have found that glioblastoma cells come in as many as four “states,” or subtypes, and – as if that were not enough – these cells can transition from one state to another. These findings might help explain why glioblastoma is so difficult to treat and point toward ways of developing future therapies.
“It’s possible that no therapy has as yet worked well against glioblastoma because each drug kills only a subset of the tumor cells, leaving others unharmed,” says Dr. Itay Tirosh, of Weizmann’s Molecular Cell Biology Department, who headed the research team with Prof. Mario L. Suvà, of the Massachusetts General Hospital (MGH) and Broad Institute. “A successful future therapy will need to attack all the four cellular states we have defined.” The research was co-led by Julie Laffy of Weizmann, Cyril Neftel and Mariella Filbin of MGH, and Toshiro Hara of the Salk Institute for Biological Studies, La Jolla, California.
Scientists have known for quite some time that cancerous tumors, and glioblastoma in particular, contain different types of cells; but existing tools provided only bulk information about the average expression of genes in a tissue sample, not within each cell individually. In the new study, the researchers resorted to advanced technology called single-cell RNA sequencing, which – as its name suggests – supplies information about gene expression in individual cells.

The researchers obtained tumor samples from 28 patients with glioblastoma – 20 adults and eight children – and created profiles of gene expression in some 24,000 tumor cells. Using specially created algorithms, they were able to correlate gene expression patterns with cell division and other normal and aberrant cellular activities. This analysis enabled the scientists to identify four different cell subtypes (“states”), each characterized by its own gene activation program. The origin of one of the programs is still unknown, but the other three are normally used by cells in the developing brain – two of them, by stem cells differentiating into more mature cell types.
Glioblastoma cells did indeed change their identity over time
“Cancer cells hijack gene activation programs of cells in the stage of growth and development, and these programs drive the growth of the tumors,” Tirosh says.
Because some cells in the study were found to simultaneously express the programs belonging to two cellular states, the researchers hypothesized that these cells were in the process of transitioning from one state to another. They designed several experiments to test this idea. In one such experiment, they “barcoded” glioblastoma cells in mice and tracked them over time, demonstrating that tumor cells indeed transition between different cellular states. In another experiment, they implanted glioblastoma cells from human cancer patients into mice. Even though these cells belonged to a particular state, they later developed into tumors containing the full spectrum of cellular states seen in the original cancer. In other words, glioblastoma cells did indeed change their identity over time.
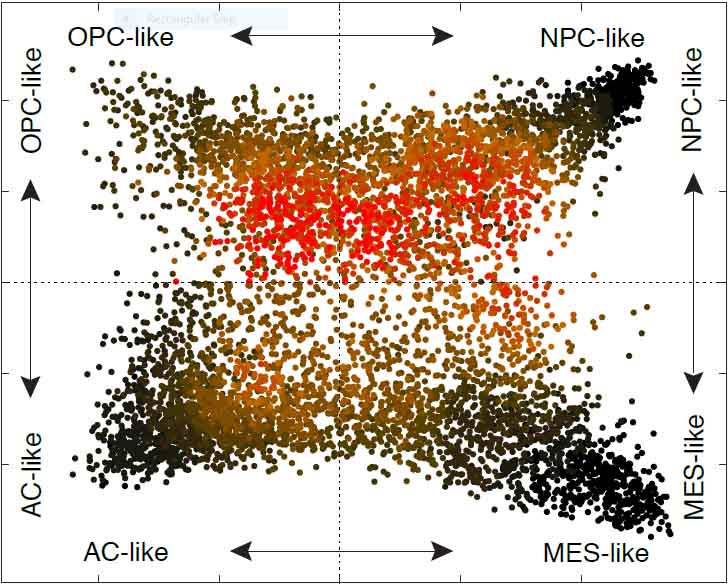
Even though all the tumors in the study contained more than one cellular state, and most contained all four, one state was usually much more abundant than the others. The scientists found that this had to do with the genetic mutations found in each tumor. They analyzed data on glioblastoma from a large database known as The Cancer Genome Atlas and identified the genetic mutations that were most common in each cellular state.
The precise characterization of glioblastoma provided by the study points the way toward targeting future therapies against all the tumor subtypes, after determining how each subtype responds to drugs. The study findings can also be applied to characterizing the subtypes that occur in other cancers.
Dr. Itay Tirosh's research is supported by the Rising Tide Foundation; the Mexican Friends New Generation Grant; the Abisch Frenkel Foundation for the Promotion of Life Sciences; the Zuckerman STEM Leadership Program; the A.M.N. Fund for the Promotion of Science, Culture and Arts in Israel; and the estate of Dr. David Levinson. Dr. Tirosh is the incumbent of the Dr. Celia Zwillenberg-Fridman and Dr. Lutz Zwillenberg Career Development Chair.