Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
One of the signs of neurodegenerative diseases such as Alzheimer’s, Parkinson’s or Huntington’s is the appearance of clumps of misfolded proteins in the nerve cells. According to new research at the Weizmann Institute, the best way to break up these clumps could be to wash them out with some “soap and water.”
Dr. Maya Schuldiner of the Molecular Genetics Department explains that these clumps, called inclusion bodies, can show up in any cell when the stress of everyday life produces too many proteins that are misfolded, unfolded or simply used up. This can happen if the “cleanup” crew – comprised of proteins called chaperones – stops working optimally, as it often does in old age. When too many proteins join these aggregates, they can interfere with normal cell activities. So the question is not only how inclusion bodies form, but how the healthy cell normally gets rid of these intrusive lumps of waste protein.
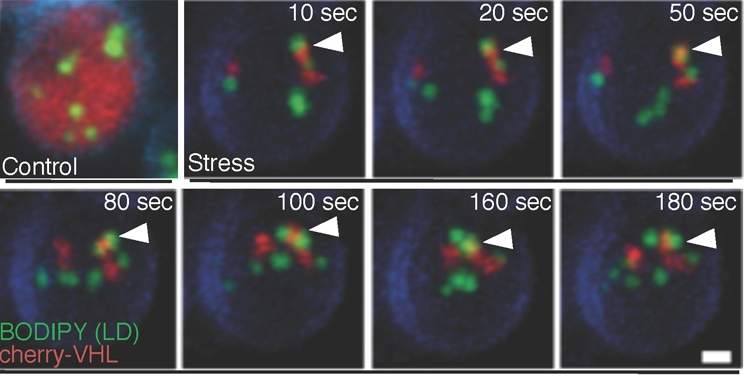
To answer this question, Schuldiner and research student Ofer Moldavski, working with the research group of Prof. Daniel Kaganovich at the Hebrew University of Jerusalem, looked at baker’s yeast cells, which, just like human nerve cells, can form inclusion bodies when cleanup fails due to age or stress. Using experimental techniques developed in her lab, Schuldiner and her team examined yeast cells they had engineered to contain human protein inclusion bodies. The inclusion bodies were tagged with a red fluorescent marker, while each of the 6,000 proteins in the yeast cell was tagged in green. This enabled the researchers to identify any yeast protein that was active around the inclusion body. The results of this research recently appeared in Developmental Cell.
Out of the 6,000, the team found 13 proteins that were attached to the inclusion bodies. Around half were previously known chaperones and the others were completely new. The next question was whether any of the newly identified proteins was actually there to clear away the waste protein clumps. Once again the team introduced the human inclusion bodies into yeast cells, making them for just a short time while knocking out, one by one, the genes for the 13 proteins they had identified. This enabled them to observe whether these proteins hindered or enhanced the clearing process.
Indeed, several of the newly found proteins were essential for clearing the inclusions – in their absence inclusion bodies stuck around, mucking up their cells. The group then chose to focus on one particular protein called Iml2. This protein has been conserved throughout evolution from yeast to humans – an indication that it plays a fundamental role in the cell. Now the question was: What exactly is that role?
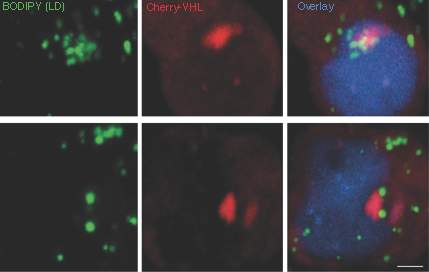
The team then repeated the experiment in human cells. Here again they observed the tethering of lipid droplets to inclusion bodies; once again this tethering was dependent on cholesterols in the lipid droplet. Schuldiner points out that in the brain cells of humans with neurodegenerative diseases, inclusion bodies had been observed cozying up to lipid droplets, but until now it was not clear why.
Schuldiner is hopeful that this newly discovered mechanism will prove to be useful in treating human disease. “Yeast cells and human cells alike turn out to be good at clearing inclusion bodies when they are young, and they get less competent at this when they age. We think they may gradually stop producing the detergent-making protein for some reason,” she says. “Instead of the present direction in research – complex, protein-based solutions that have mostly failed in the clinic – a ‘detergent-based’ treatment might be a simple and effective ‘chemical’ remedy for such age-related, neurodegenerative diseases as Alzheimer’s and Parkinson’s.”
Dr. Maya Schuldiner’s research is supported by the Foundation Adelis; the Georges Lustgarten Cancer Research Fund; the Dora Yoachimowicz Endowed Fund for Research; the Berlin Family Foundation; Roberto and Renata Ruhman, Brazil; Karen Siem, UK; and the estate of Lola Asseoff.