From life's very beginning, when the ovum starts dividing into two primal cells, our existence depends on cell reproduction. In adults, billions of cells divide every day to replace cells that wear out or to meet a sudden demand. Yet the most critical moment, when the mother cell divides into two new daughter cells, remains a mystery.
The different stages of cell division - a process so orderly as to appear almost choreographed - have long been known. While tremendous advances in cell biology and genetics over the past few years have shed light on this process, its underlying molecular machinery is still not fully understood. The final step of this elegant performance, the separation into two cells, is called cytokinesis. Recently, Dr. Sima Lev of the Weizmann Institute of Science's Neurobiology Department discovered that a protein called Nir2 is essential for normal cytokinesis in human cells.
As a postdoctoral researcher at New York University, Lev discovered a protein called Pyk2, which plays an important role in cell signaling. Looking for proteins that interact with Pyk2, she discovered a protein family consisting of three members, which she called the "Nir"family. She then isolated the genes responsible for producing the proteins.
Highly conserved throughout evolution, the Nirs are found in fish, worms, flies, and mammals. Lev decided to dedicate her work todetermining the Nir proteins'function in the body. "No one in the world was working on the Nirs,"she says, "and I strongly believed that they had an important cellular function."
Upon her return to the Weizmann Institute, Lev spent nearly three years evaluating many possible roles for the protein, with team members Vladimir Litvak, Donghua Tian, and Shari Carmon. The breakthrough came with their identification of a particular fragment of Nir2, consisting of 219 amino acids out of the protein's full 1,244. When they expressed this fragment in human cells, it had adramatic effect on their shape and caused severe defects in cytokinesis. The cells failed to separate, forming long bridges between asymmetrical daughter cells. The fragment, Lev concluded, was inhibiting cytokinesis in some way, but its precise role remained obscure.
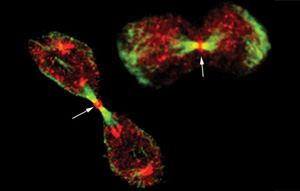
In a pinch
The scientists looked closely at dividing cells to determine exactly where the Nir2 protein was located during the process. They found that during normal cytokinesis Nir2 is present at the "cleavage furrow,"the pinched area of the cell at which the break into two daughter cells will eventually take place. But it's not alone. Beside it is an enzyme called Rho-GTPase, which plays a long-established role in cytokinesis. What, Lev wondered, was Nir2 doing there?
She found that Nir2's protein fragment is able to inhibit the activity of the Rho enzyme. She therefore designated it "Rid"(Rho inhibitory domain). It was already known that inactivation of the Rho enzyme is necessary for the final separation into two daughter cells, but it was not known what triggered Rho to move into an inactive state. Lev contends that Nir2 essentially subcontracts Rid to inhibit the activity of Rho when appropriate. If she is right, Nir2 - by hosting Rid - is vital for breaking the contractile ring between two daughter cells and thus is essential for successful cytokinesis.
The physical evidence supports Lev's assertion. When she cut off the end of Nir2 containing Rid, she saw that cytokinesis was severely impaired. The cells struggled to divide and eventually gave up, resulting in unseparated cells with multiple nuclei. The absence of Rid apparently short-circuited the cells'ability to separate.
Lev's findings shed new light on the Nir proteins as well as on the process of cytokinesis. But many open questions remain regarding the clinical implications of her results. It is well known that cytokinesis plays a critical role in animal development, and that defects in this stage of cell division can lead to instability of the genome, a phenomenon associated with cancer. In addition, recent experiments have shown that mouse embryos which lack the Nir2 protein do not survive. Thus her findings may provide insights into the necessity of this protein for normal embryonic development. "The challenge now,"says Lev, "is to translate these results into practical medicine."
Dr. Lev's research is supported by Mr. and Mrs. Nathan Baltor, Bensalem, PA; Minna James Heineman Stiftung, Germany; the Carl and Micaela Einhorn-Dominic Institute for Brain Research; and the Nella and Leon Benoziyo Center for Neurosciences. She holds the Helena Rubinstein Career Development Chair.

