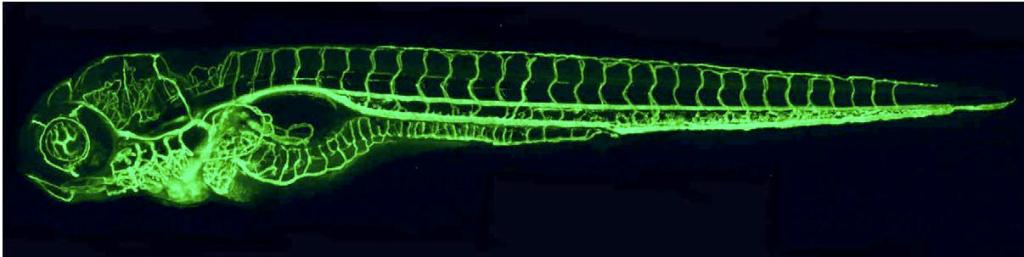
Blocked or constricted blood vessels lead to high blood pressure, heart attack and stroke – some of the most common causes of death in the Western world. Blood vessels become blocked when fat is deposited on artery walls, building up until the flow of blood is restricted and the delivery of oxygen and nutrients curtailed. Today, such “clogged pipes” are cleared with drugs, angioplasty or bypass surgery to replace the blocked sections. But in the lab of the Weizmann Institute’s
Dr. Karina Yaniv, researchers are looking for ways to keep the body’s main conduits from getting clogged up in the first place.
Type your text here
Type your text here
Type your text here
To prevent blockages, one must understand exactly how they occur. As opposed to the kitchen drainpipe, which gets clogged from the passive accumulation of gunk, the deposition of fat on blood vessel walls is an active process in which the cells lining those walls can sense fats carried in the bloodstream and respond according to amount and type. According to Yaniv, that fat and those vessel-lining cells – endothelial cells – engage in a two-way conversation that facilitates the fat build-up. Basically, the endothelial cells give the fat permission to cross the inner layer of the “pipe” to settle comfortably on the other side of that layer. As the fat layer grows, it presses on the blood vessel walls, constricting them and creating the conditions for blood vessel disease.
In
research that appeared in
Nature Medicine, Yaniv and her team in the Biological Regulation Department revealed how fat levels in the blood act on the endothelial cells. To travel in the bloodstream, fats are packaged into units along with proteins; these hold the fats and help them enter into the body’s cells. These units, called lipoproteins (fat-proteins) also contain cholesterol – either the “bad” cholesterol (LDL) or the “good” cholesterol (HDL). The research focused on units made with bad, LDL, type. Such lipoproteins have large fat-to-protein ratios, and they are known to be high risk factors for the development of blood vessel disease. “Medicine is concerned with the fat content of the LDL lipoprotein, but we showed that the protein component of this unit plays a crucial role in the conversation with the endothelial cells,” says Yaniv.
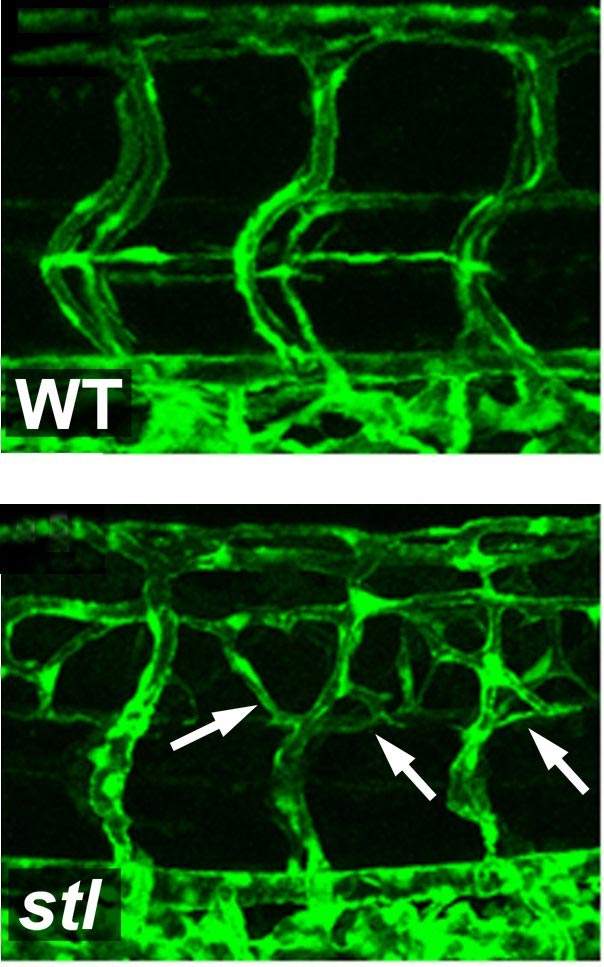
Working with zebrafish embryos, the research team first discovered a genetic mutation that causes the overproduction of blood vessels – almost twice the normal number. Their research showed that the mutated gene is responsible for the packaging and secreting of the LDL: It ties the fat molecules up with the transport protein, called ApoB, and then sends the packaged unit off into the bloodstream. When this gene was damaged in the fish, their bodies didn’t produce the bad cholesterol. A similar mutation exists in certain humans. Carriers of this mutation don’t produce LDL, and they don’t suffer from such fat-related blood-system diseases as arteriosclerosis.
How does LDL or its lack affect the growth of new blood vessels? The researchers found that lowering the bad cholesterol levels resulted in an increased division of the endothelial cells, while raising LDL levels had the opposite effect: Cell division was delayed, as was the cells’ ability to migrate and contribute to the formation of new blood vessels. Further investigation revealed that LDL directly interferes with a main mechanism of endothelial cell proliferation, in which a growth factor called VEGF binds to the cell surface to activate the process. VEGF can bind to two different types of receptors on the cell. The first is the “regular” receptor, which responds to VEGF binding by initiating cell proliferation. The second is a “dummy” receptor, which binds but does not initiate division. The dummy receptors are a sort of double-check and regulation mechanism, but LDL appears to encourage the production of this type of receptor. Thus high LDL levels mean more of the dummy receptors and less endothelial cell proliferation, while low levels promote cell division and, thus, the creation of new blood vessels.

It is the ApoB protein, which is packaged together with the cholesterol and triglycerides, that seems to act as a mediator between the lipoprotein units and the endothelial cells, and so its function is central to the formation of blockages. Understanding the mechanism by which ApoB and LDL contribute to endothelial cell damage may enable us, in the future, to adjust the process, possibly by promoting the production of new blood vessels to bypass the blocked sections.
Dr. Karina Yaniv's research is supported by the Karen Siem Fellowship for Women in Science; the Willner Family Center for Vascular Biology; the estate of Paul Ourieff; the Carolito Stiftung; Lois Rosen, Los Angeles, CA; the Adelis Foundation; and the Yeda-Sela Center for Basic Research. Dr. Yaniv is the incumbent of the Louis and Ida Rich Career Development Chair.