Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Like the swift and loyal messenger who rushes to deliver the king’s missive in a fairy tale, a string of RNA called messenger RNA, or mRNA, appears in the oft-told story of the living cell. This RNA, copied from the DNA in a gene, had been thought to be immediately sent from the cell nucleus to the body of the cell – the cytoplasm – where the protein factories await its message. But a surprising new Weizmann Institute study suggests that certain mRNAs may be less like fabled fleet couriers and more like certain drivers at the local taxi stand – hanging out at home base and drinking coffee for a good part of the morning before heading out. This research, which was reported recently in Cell Reports, adds a new twist to the well-known story of how our genes are translated into all the proteins in our bodies.
Dr. Shalev Itzkovitz and his group in the Molecular Cell Biology Department have developed a unique system, combining advanced microscopy with computational approaches, which enables them to visualize individual RNA molecules – of any type – in tissue samples. The group used this system to look into the production of an important protein in the liver. When they checked their results, the researchers did a double-take: the RNAs for this protein were mostly congregated within the nucleus, rather than off in the cytoplasm, where all protein production takes place.
Why would mRNA “hang out” inside the nucleus? The group, including Dr. Keren Bahar Halpern and Inbal Caspi in Itzkovitz’s lab, decided to investigate. A check of all the liver RNA in nuclei and cytoplasm suggested that around 15% of all the liver genes give rise to RNAs that loiter in the nucleus. Likewise, in the beta cells in the pancreas, 30% of RNA was found to hang around in the nucleus, including some that was crucial to the cells’ function.
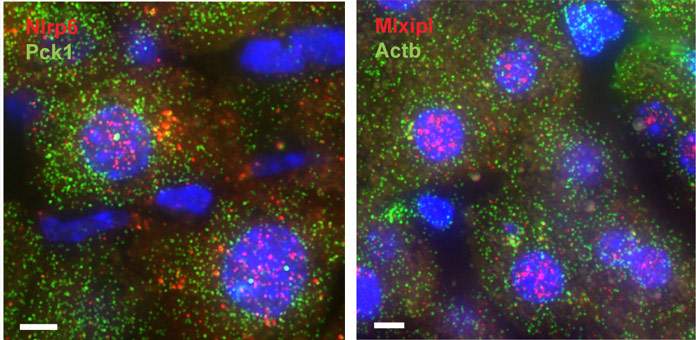
How much time do the “homebody” RNAs spend hanging out in the nucleus before heading off to the cytoplasm to do a bit of work? The researchers developed a technique to answer this question as well for some of the key genes: one hour, on average, followed by a much shorter lifetime of around 30 minutes in the cytoplasm.
One possible explanation for RNA loitering in the nucleus is that it could be a sort of emergency reserve. This was suggested by, among other things, one of the sequences the team identified that participates in an immune response. But when the scientists tried exposing the tissue to different conditions that might require larger amounts of these proteins, they found that the levels of the RNA within the nucleus stayed pretty much the same.
They then turned to a second theory, which suggested that this extra RNA serves as a “buffer” between the activities of the DNA and that of the protein manufacturing centers in the cytoplasm. Previous research in Itzkovitz’s lab and others had shown that RNA is copied from the DNA in irregular bursts as the DNA double helix unwinds and rewinds. This means that each nucleus is producing different amounts of RNA, while the cells need to work together as a team. Keeping the RNA in the nucleus and letting it out bit by bit in “time release” could be a way of coordinating protein production and overcoming the “noise” of the “bursty” DNA.
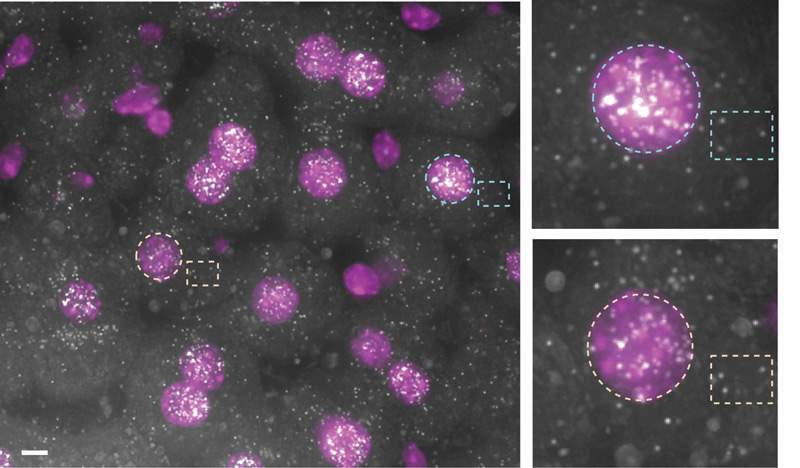
The team developed a mathematical model showing that this may, indeed, be the reason behind the RNA retention in the nucleus. Further testing showed that the distribution of the homebody RNA in neighboring liver cells varied from nucleus to nucleus but was fairly uniform outside the nuclei – supporting this idea.
Itzkovitz believes that these findings will open a number of new avenues in RNA research. “Because the phenomenon we discovered is so widespread, we think it may have functions beyond buffering the DNA ‘noise’,” he says. “For example, it may facilitate the rapid response of cells by regulating the release of the nuclear mRNA in response to diverse stimuli, thus shortening the response time that is associated with the slow transcription process. This theory and many others remain to be tested.
Dr. Doron Lemze and Shanie Landen of the Molecular Cell Biology Department; Dr. Eran Elinav and Maayan Levy of the Immunology Department; and Dr. Igor Ulitsky of the Biological Regulation Department participated in this research.
Dr. Shalev Itzkovitz's research is supported by the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics; the Leir Charitable Foundations; the Richard Jakubskind Laboratory of Systems Biology; the Lord Sieff of Brimpton Memorial Fund; and the European Research Council. Dr. Itzkovitz is the incumbent of the Philip Harris and Gerald Ronson Career Development Chair.