Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Even after prevailing over a viral infection, our immune system stays active, protecting us from any lingering viruses or recurring disease. But what happens if we pick up a bacterial infection – say, salmonella food poisoning from take-away chicken soup – while recovering from flu or COVID-19? A new study at the Weizmann Institute of Science published today in Immunity, shows that in such cases, the immune system has a clever way of setting priorities, one that might be exploited for the development of future therapies against autoimmune diseases.
This prioritizing involves the two arms of immunity: innate and adaptive. Innate immunity, the body’s first line of defense, springs into action as soon as the immune system senses an invasion by viruses, bacteria or other pathogens, deploying its cells and biochemicals in a broad offensive to neutralize the invaders. Adaptive immunity, on the other hand, may take several days to roll out its weapons: dedicated cells, as well as antibodies that are tailored to bind to different invaders with exquisite precision. The antibodies stay around for months or even years, offering long-lasting security.
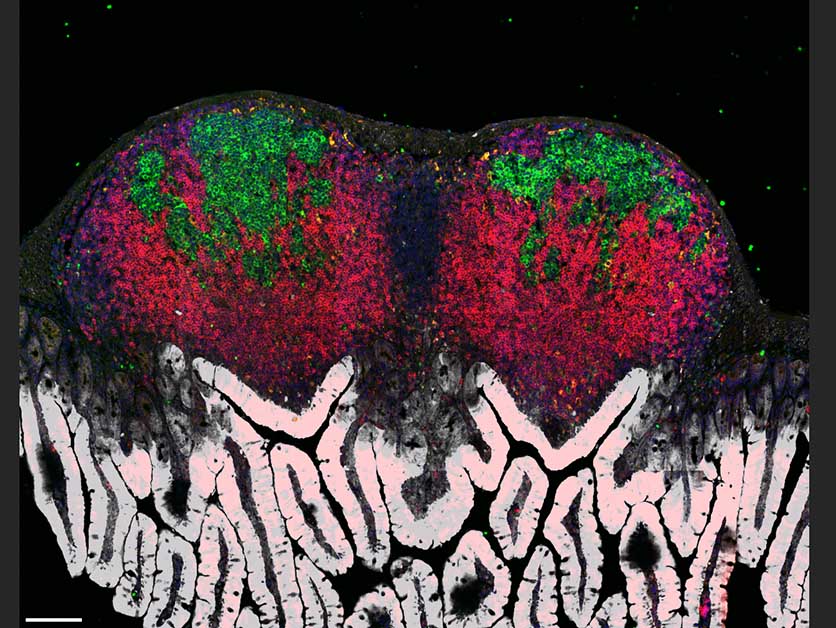
This means that innate and adaptive immunity generally come to the fore at different stages of an infection. But when one infection is followed by another – for example, when a person overcoming a flu virus comes down with a bacterial infection such as salmonella – the two arms are forced to go into high gear at the same time: Innate immunity starts fighting the bacteria while adaptive immunity is still busy making antibodies against the flu virus.
A team headed by Prof. Ziv Shulman of Weizmann’s Immunology Department set out to learn how the two arms of immunity interact during such an overlap. In a study using mice, led by PhD student Adi Biram, they found that the interaction ends in a clash: Infection with salmonella interferes with the manufacture of antibodies against the flu. In other words, when faced with a lethal threat, the immune system shuts down mechanisms needed for long-term protection, dealing instead with the more urgent danger.

The researchers revealed that salmonella doesn’t produce this effect directly. Rather, when it infects lymph nodes, it sets off an alarm that reaches as far as the bone marrow, priming innate immunity cells called monocytes to rapidly leave the marrow in order to fight the bacteria. These monocytes flood the lymph nodes en masse, from there launching an attack on the salmonella. But in the process, as a result of their antimicrobial activity, these cells alter the environment within the lymph nodes, releasing various chemicals and causing a local oxygen shortage.
""It’s an either/or situation – when you are fighting life-threatening bacteria, you can’t be bothered with long-lasting immunity"
Most immune cells adapt to this shortage by changing their metabolism, shifting to burning glucose for energy instead of oxygen. But for a subset of B cells that reside in the lymph nodes in microscopic structures called germinal centers, an oxygen shortage proves fatal: Unable to adapt their metabolism, these B cells choke and die. This is precisely the subset of cells that plays a key role in adaptive immunity, generating antibodies having the best possible fit against the invading pathogen. The death of these cells puts an end to the production of antibodies required for long-lasting protection against the viral infection.
“It’s an either/or situation – when you are fighting life-threatening bacteria, you can’t be bothered with long-lasting immunity,” Shulman explains. “Destroying the salmonella gets priority because it’s a matter of survival.”
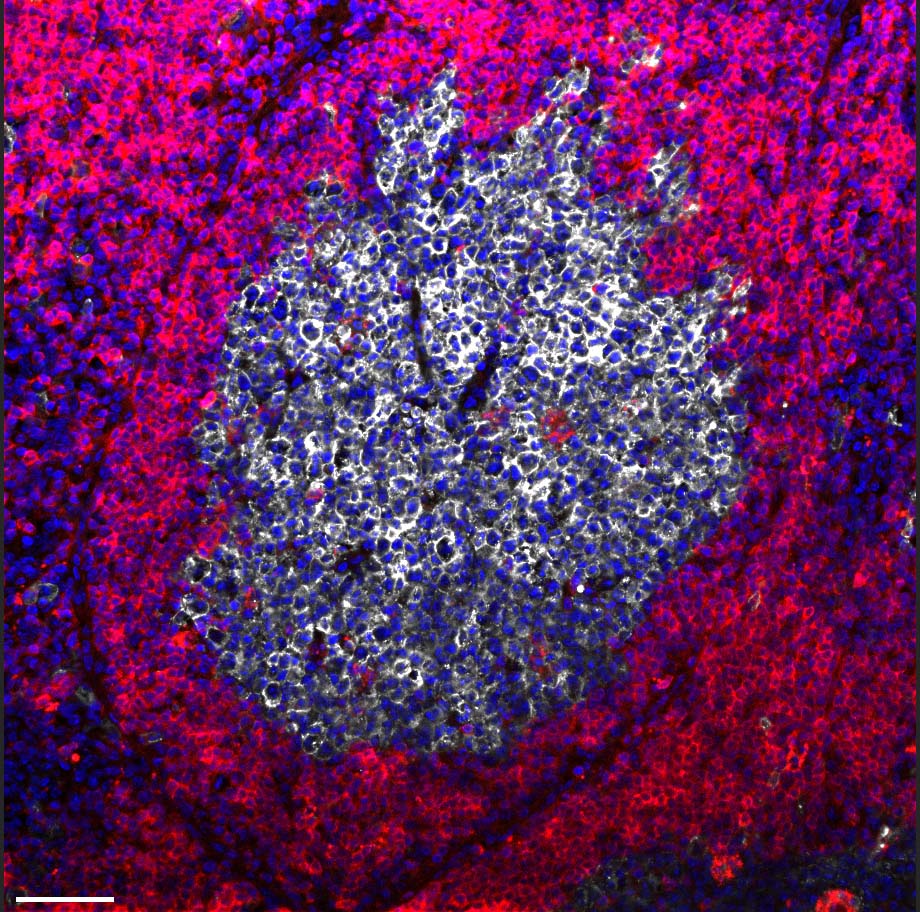
The study’s findings may have applications in various areas of immunology. Currently, certain bacterial proteins are sometimes added to vaccines in order to enhance their effectiveness, but the study suggests that such additions might backfire, harming antibody production. Moreover, if confirmed in humans, the new findings might lead to a new type of therapy for autoimmune diseases caused by the mistaken production of antibodies, for example, rheumatoid arthritis and lupus. Such a therapy would harness bone marrow monocytes to stop the production of disease-causing antibodies.
Also taking part in the study were Jingjing Liu, Dr. Hadas Hezroni, Dr. Natalia Davidzohn, Dr. Dominik Schmiedel, Dr. Eman Khatib-Massalha, Montaser Haddad, Dr. Amalie Grenov, Prof. Tsvee Lapidot and Prof. Steffen Jung of the Immunology Department; Sacha Lebon, Dotan Hoffman and Dr. Roi Avraham of the Biological Regulation Department; Dr. Tomer Meir Salame of the Life Sciences Core Facilities Department; Dr. Nili Dezorella of the Chemical Research Support Department; Paula Abou Karam of Biomolecular Sciences Department; and Prof. Mats Bemark of the University of Gothenburg, Sweden.
90% of deaths that occurred during the 1918 influenza pandemic are thought to have resulted from secondary pneumococcal pneumonia. In a large study at Rabin Medical Center, secondary bacterial infections occured in 12.6% of patients with COVID-19 and in 8.7% of flu patients.
Prof. Ziv Shulman’s research is supported by the Moross Integrated Cancer Center; the Rising Tide Foundation; the Azrieli Foundation; the Ben B. and Joyce E. Eisenberg Foundation; the Wolfson Family Charitable Trust & Wolfson Foundation; Elie Hirschfeld and Dr. Sarah Schlesinger; and Miel de Botton.