Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
With hundreds of millions of diabetics around the world and no cure for the disease, researchers have taken numerous tacks to find better treatments. One promising approach that may be used in the future is cell reprogramming – getting some of the cells neighboring the dysfunctional, insulin-producing beta cells to take up the slack. Such cells have been reprogrammed in the lab, but success has been limited. New research at the Weizmann Institute of Science identifies a natural roadblock to reprogramming that impairs the efficient generation of new beta cells.
Prof. Michael Walker of the Institute’s Biomolecular Sciences Department explains that there are two major forms of diabetes: type 1, which generally is diagnosed in children and in which the beta cells are attacked by the body’s immune system; and type 2, in which a person’s beta cells gradually succumb to an insulin-demand overload. Oral medications and insulin injections treat the symptoms of the disease, but there is still no cure, and the disease often progresses despite treatment, leading to serious complications. One experimental treatment for type 1 diabetes in recent years involves transplanting beta cells from organ donors. Unfortunately, there are not enough cells from organ donors to go around, and just as with any organ transplant, the recipient must take immunosuppressant drugs to prevent rejection.
When the researchers kept this transcription factor from functioning, they observed much more efficient reprogramming
Another finding of recent years has, however, brought new hope: researchers have found that cells are not as “fixed” in their final forms as was once thought. In many labs researchers have learned to take cells all the way back to their origins, turning them into stem cells, but others, like Walker and his group, have been investigating the possibility of nudging similar cells, for example the exocrine cells in the pancreas that produce digestive enzymes, into retooling their “production lines.” These cells make up some 98 percent of the secretory cells in the pancreas, so only a few would need to switch their roles. And since they are, in both their location and their developmental origin, similar to the beta cells, the researchers believe that switching just a handful of genes on or off would be enough to make the change.
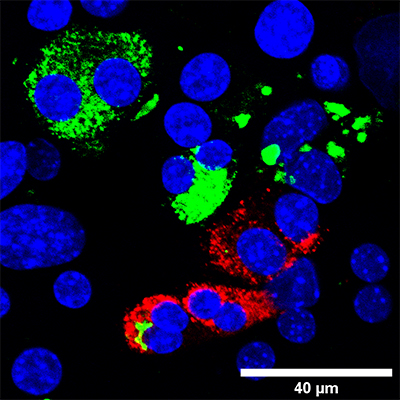
Dr. Ofer Elhanani, a postdoctoral fellow in Walker’s group, led the current study. He has been attempting to understand the process of beta-cell reprogramming, which, like much cell reprogramming until now, has been a mysterious process in which researchers insert genes into adult cells and await an outcome. Walker, Elhanani and the lab team grew exocrine pancreatic cells in the lab and inserted transcription factors – molecules that turn groups of genes on or off – into the cells. These transcription factors are associated with the insulin-producing functions of beta cells. Then, when some of those cells showed signs of transitioning into beta cells, the researchers separated their cells into two groups and looked for differences.

Why did some cells transform while others didn’t? Walker, Elhanani and their team first had to develop a way of identifying those cells that were, after several days in the lab, undergoing the transformation; they then needed to find a way of separating them from the cells that were stubbornly refusing to change. They accomplished this by adding a fluorescent label that would tell them when a cell had insulin-production abilities, and they then used a method called flow cytometry to pick out individual cells that glowed. When they compared these two groups, they discovered the presence of another transcription factor, called REST, which is normally expressed in the exocrine pancreatic cells but not in beta cells. When the researchers kept this transcription factor from functioning, they observed much more efficient reprogramming.
Probing further, the team discovered how REST prevented the expression of key beta cell genes: It attaches to certain crucial genes in the cells, close to the sites to which the beta-cell transcription factors need to bind, thus blocking their activity and silencing the genes.
“If we want to efficiently reprogram cells, we will first need to understand the molecular details of the process and the natural barriers that block this process from occurring in normal cells,” says Walker. “Identifying REST as such a barrier will provide us with new tools for creating insulin-producing cells.” “Of course, there will be many hurdles in bringing such research from the lab to the clinic, but discovering why something does not work as expected is the first step toward fixing it,” adds Elhanani.
Also participating in this research were Dr. Jonathan Sobel, Lital Povodovski and Itay Vaknin of the Institute’s Biomolecular Sciences Department, Drs. Tomer Meir Salame and Dena Leshkowitz of the Life Sciences Core Facilities; and Dr. Dror Kolodkin-Gal of Hadassah–Hebrew University Medical Centre, Jerusalem.
Prof. Michael Walker's research is supported by the David and Fela Shapell Family Center for Genetic Disorders Research; the Kekst Family Institute for Medical Genetics; the Dr. Beth Rom-Rymer Stem Cell Research Fund; the Horowitz/ Kaufman Diabetes Research Fund; Florence Blau, Morris Blau and Rose Peterson Fund; and the estate of Albert Engleman. Prof. Walker is the incumbent of the Marvin Myer and Jenny Cyker Professorial Chair of Diabetes Research.