Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
How do neurons decide when to grow and when to stop growing? In a new study reported in Cell, Weizmann Institute scientists found a switch that turns neuronal growth on or off. This research helps explain how the brain is wired with such great precision during development; in the future, it may provide clues to how neurons deteriorate in Alzheimer’s and other neurodegenerative diseases, or what prevents neuronal regeneration after brain or spinal cord injury.

As the brain develops, certain neurons at first grow extensions abundantly; they then cause some of these extensions to degenerate in a process known as “pruning.” Later on, these extensions may regrow to help wire the adult brain. The team of Dr. Oren Schuldiner in the Molecular Cell Biology Department – research student Dana Rabinovich and staff scientist Dr. Shiri Yaniv together with Idan Alyagor – studied neuronal growth in a unique system previously designed in Schuldiner’s lab: the brain of a fruit fly pupa that continues to develop in a laboratory dish after being removed. When the scientists spiked the dish with a chemical that lowered the levels of nitric oxide, NO, they realized that these neurons sprouted new extensions faster than those in the control brains. By genetically manipulating the developing fruit flies, the researchers then confirmed that NO acts as a switch for neuronal growth in living flies. NO is a widely studied signaling substance that was even named “molecule of the year” by Science magazine in 1992, but the recent Weizmann findings revealed a previously unknown biological mechanism for this substance.
It is apparently the changes in NO levels that enable the neurons to switch from degeneration to regrowth within a few short hours; having such a switch ensures that these activities occur at different times, never interfering with one another. When NO levels are low, the neuron grows its extensions, while just the opposite occurs when NO levels are high – existing neuronal extensions undergo pruning. In fact, NO seems to be essential for pruning; this process fails to take place when NO is not produced in sufficient amounts.
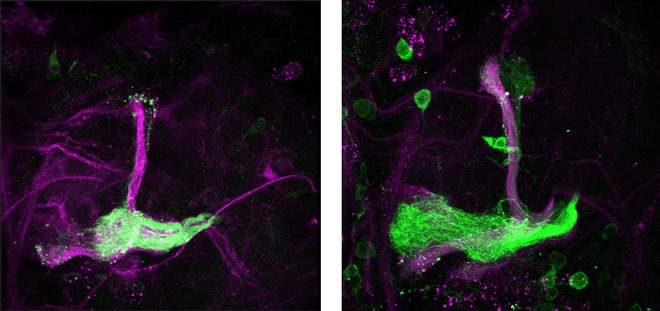
In further experiments, the scientists determined that the neurons themselves produce the NO. They also unraveled the mechanism by which the NO switch works in neuronal regrowth in the developing brain. High NO levels block the activity of a receptor that is essential for regrowth; but when these levels drop, the receptor is free to join up with yet another molecule, forming a complex that sets regrowth in motion.
These findings open up a new avenue of research into neurodegenerative diseases and into neuronal regeneration after injury in humans. Indirect evidence indeed suggests that NO may be relevant to both situations. For example, high NO levels have been measured in the brains of people with Alzheimer’s and Parkinson’s disease. In addition, the same chain of biochemical events that was found to be controlled by NO in this study had been previously shown to be important for regeneration after injury.
Pruned Neurons Must Come “Unglued” In separate research Dr. Oren Schuldiner and his team identified a previously unknown step in the remodeling of neuronal circuits of the developing brain. Graduate student Bavat Bornstein and other team members focused on axons, the long neuronal extensions that transmit information to other neurons and muscles. Bornstein systematically examined the genes of the fruit fly, whose neuronal circuits undergo massive remodeling when its larva metamorphoses into an adult organism. The team used a screening approach developed by Schuldiner to identify functions of the fly’s genes by creating various mutations and observing their consequences. As reported recently in the journal Neuron, the screening revealed that the pruning of axons in the fly cannot proceed without a gene called Bsk. When this gene was mutated, no pruning took place. Remarkably, Bsk was shown to be essential for the pruning of axons, but not of other neuronal extensions. The scientists then found that Bsk exerts its effect on pruning by regulating cell adhesion. It reduces the stability of a key adhesion molecule, that is, one that “glues” the axons together into a bundle. In other words, for the pruning of the axons to take place, the axons first need to become “unglued.” This discovery, in turn, points to previously unknown mechanisms of neuronal remodeling. It raises the intriguing possibility that the same genes and molecules that control the “stickiness” of neuronal axons also determine where and when the axon must undergo pruning. 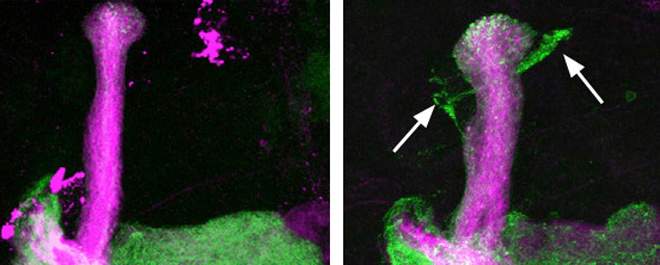 Mammals have an equivalent of the Bsk gene, called JNK. Known to affect a wide variety of cellular processes, JNK serves, for instance, as an oncogene in cancer cells and controls neuronal cell death in the nervous system. Now the new study suggests an entirely new role for this gene, opening a new direction of research into axonal pruning and brain remodeling during development. Moreover, because axonal pruning in many ways resembles the disintegration of axons that takes place in neurodegenerative diseases, yet another potential new avenue of research would be to check whether the molecules that affect the “stickiness” of axons play a role in these disorders. Study authors included Sivan Gelley, Maayan Zoosman, Dr. Shiri Penina Yaniv and Ora Fuchs from Schuldiner’s lab, as well as Dr. Ziv Porat of Weizmann Institute’s Biological Services Department, and Eitan Erez Zahavi and Dr. Eran Perlson of Tel Aviv University’s Sackler Faculty of Medicine.
|
Dr. Oren Schuldiner's research is supported by the Adelis Foundation. Dr. Schuldiner is the incumbent of the Aser Rothstein Career Development Chair.