Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
The cells in our bodies must constantly communicate with one another. For many, it is a matter of survival; for others, it is the way they keep our bodies healthy and functioning efficiently. Communications are carried out by proteins – both the numerous proteins that are situated on the cells’ outer membranes to receive the messages and the messengers themselves, which are secreted to the outside of the cell. For most of these proteins, getting to the outside of the cell involves passage through an organelle called the endoplasmic reticulum. This first entails getting across the membrane of this organelle, to which the proteins are targeted, with the help of a special “shuttle” that conducts them to a sort of “transit area,” checking them first to see if they have the proper “passports.”
How many different shuttles are needed to move all these proteins – in effect, around 30% of all the proteins in each cell? Previous studies of the past few decades have identified two – sort of couriers, like FedEx or DHL for proteins. Now, a new study, conducted in the lab of Prof. Maya Schuldiner of the Institute’s Molecular Genetics Department, has uncovered a third shuttle, and raised the possibility that more might be awaiting discovery.

The study was led by research student Naama Aviram, in collaboration with the labs of Prof. Richard Zimmerman of Saarland University, Germany, Prof. Blanche Schwappach of Göttingen University, also inGermany, and Prof. Jonathan Weissman of the University of California, San Francisco. “The first pathway for transferring proteins into the endoplasmic reticulum was discovered in the 1980s,” says Schuldiner. “Scientists found that this shuttle, called SRP, identifies the protein to be transported by reading a tag that is a sort of ‘passport’.”
But this finding was not the whole story: Although many proteins use the SRP pathway to get to the endoplasmic reticulum membrane, this shuttle system seemed to have trouble identifying other outward-bound proteins. The reason eventually became clear: SRP easily identifies the tag when it is situated at one end of the protein, but has a hard time with tags at the other end. “This suggested that there was another pathway to catch the proteins that SRP misses,” says Schuldiner. “We identified that pathway in 2008 and named it GET.”
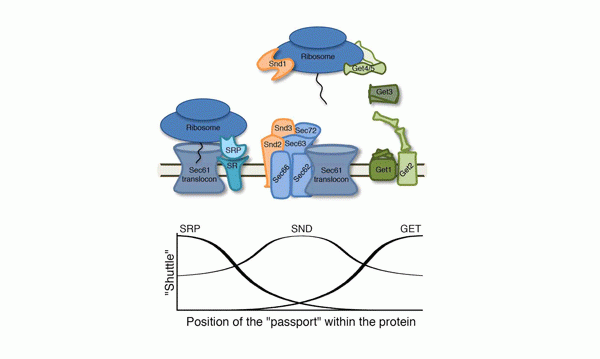
But even with two shuttle services, the scientists noted there were still proteins that were not easily recognized by the pathways. “These are proteins that, if they are not efficiently transported to the endoplasmic reticulum, the cell dies. So we undertook a search for yet another pathway,” says Aviram.
The team began their experiments in yeast cells, whose basic functions are nearly identical to those of human cells, and then tested their results in human cells. To begin, the researchers identified proteins that need to pass through the endoplasmic reticulum transit area, but do not receive assistance from either known pathway.
Then, using the advanced robotic system in Schuldiner’s lab, they systematically looked at the ability of a protein to reach the endoplasmic reticulum membrane in the absence of each gene in the yeast cell, finding three that appeared to be necessary to the process of transporting these particular “problematic” proteins. When these genes were missing, the researchers observed accumulations of protein within the cell – proteins that had not managed to reach their destination outside of it.
How many different shuttles are needed to move all these proteins – in effect, around 30% of all the proteins in each cell?
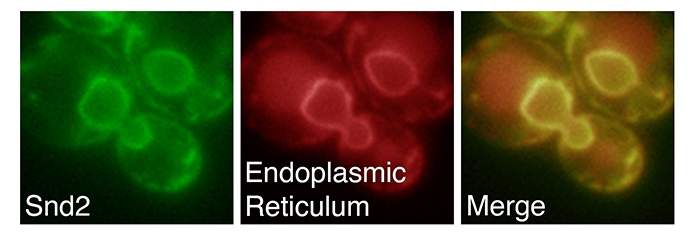
The three genes, which the team called SND1, SND2 and SND3, work together; one on the ribosome (the cell’s protein-manufacturing complex) and the other two at the “gates” of the endoplasmic reticulum.
Together with the Weissman lab in San Francisco, the scientists revealed that this third pathway is active when the “passport” is closer to the center of the protein – the region the other two pathways have a hard time reading. “The new pathway functions as a ‘safety net’ for crucial proteins that may need to catch the next shuttle, but have their tags in inconvenient places,” says Schuldiner.
Together with the Zimmerman lab, the researchers then asked whether this was occurring in a similar way in human cells. The scientists silenced the human SND2 gene – which they found has been conserved throughout evolution – and showed that here, too, the passage into the endoplasmic reticulum was defective, suggesting that this third pathway is at work in human cells as it is in yeast.
“Many diseases, for example diabetes, involve disruption to intercellular communications,” says Schuldiner. “And we don’t always know just where the message goes astray. Maybe, in the future, understanding this pathway might help us figure out how to treat disease and save lives.”
Prof. Maya Schuldiner's research is supported by the Foundation Adelis; the Georges Lustgarten Cancer Research Fund; the Dora Yoachimowicz Endowed Fund for Research; the Berlin Family Foundation; amd Roberto and Renata Ruhman, Brazil. Prof. Schuldiner is the incumbent of the Dr. Gil Omenn and Martha Darling Professorial Chair in Molecular Genetics.