Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Border controls are the way a country regulates who and what crosses into its territory. Living cells also have "border controls" – membranes that surround the whole cell or especially sensitive or important parts of it. One of the cell's organelles – the nucleus – is even enclosed in a double membrane that serves to protect its highly valuable contents – the organism's genetic material. This membrane regulates the flow of molecules into and out of the nucleus via "border gates" – referred to as membrane-bound pores – helping to prevent erroneous DNA activation.
Those molecules authorized to enter the nucleus and carry out legitimate activities usually possess a special "localization code" – a specific amino acid sequence harbored within a particular region of the molecule. This code is recognized by special carrier proteins (importins), which then facilitate the transport of these molecules into the nucleus.
Scientists have only recently begun to notice that some molecules entering the nucleus lack the localization code. So how do they pass through the border? In a research article recently published in Molecular Cell, Prof. Rony Seger and Ph.D. student Dana Chuderland, with help from postdoctoral fellow Dr. Alexander Konson, all of the Weizmann Institute's Biological Regulation Department, identified a previously unknown mechanism that grants permission to some of these molecules to enter. Because certain molecules that cross over into the nucleus are overactive in such diseases as cancer, this new mechanism may present an effective means of "stopping them at the border."
Seger's team focused on signaling proteins named ERK. These proteins, which function mainly within the nucleus, are involved in such cellular activities as gene expression (the production of proteins) as well as cell proliferation and differentiation. Seger's group discovered, through experiments and bioinformatics analyses, that these signaling proteins do possess a localization code after all – but its sequence is different from that of the commonly recognized one. As opposed to the molecules possessing the regular localization code, molecules bearing the newly identified code do not have an automatic free pass; the team identified further entry criteria they need to meet. ERK proteins are usually held in "customs" – anchored to other proteins in the cell's cytoplasm – and can only get released upon extracellular stimulation of the cells with growth factors and hormones. Once this occurs, their particular entry code needs to get "stamped" with a phosphate group. (The addition or removal of a phosphate is an important regulatory mechanism of cellular activity.) Only then can these proteins bind to the particular carrier protein that helps them localize to the nucleus, where they can carry out their assignment.
When the newly discovered entry code was removed from these proteins or the addition of the phosphate groups was prevented, the ERK proteins remained stranded outside the nuclear membrane, thus confirming that both are necessary for crossing the border. The scientists noted that these events inhibited cellular proliferation, hinting that the new code might be useful for fighting cancer, in which cell reproduction goes out of control.
It turns out that ERK is not the only type of protein to use this mechanism; a bioinformatics search revealed that about 40 proteins can translocate to the nucleus by the same route. In other words, Seger's group seems to have identified a unique general mechanism shared by these proteins.
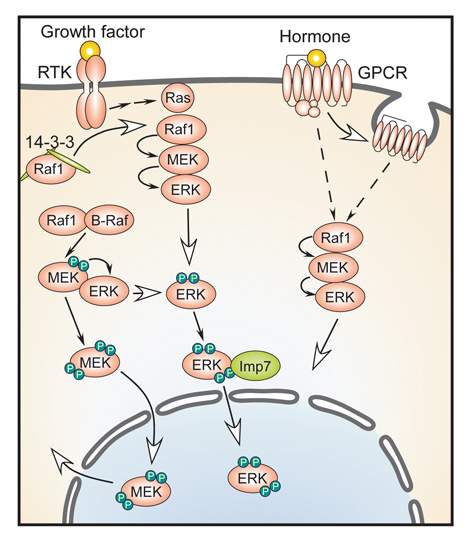
These findings may have important implications for the development of new therapies: Inappropriate activation of ERK proteins and ensuing cell proliferation is common in human cancers. Because these proteins are important in so many other processes, however, existing treatments that target all ERK proteins lead to undesirable side effects. A drug that could selectively target the newly identified entry code might act mainly to prevent proliferation, resulting in a more effective anti-cancer drug with fewer adverse reactions.
Prof. Rony Seger's research is supported by the M.D. Moross Institute for Cancer Research; and the Phyllis and Joseph Gurwin Fund for Scientific Advancement. Prof. Seger is the incumbent of the Yale S. Lewine and Ella Miller Lewine Professorial Chair for Cancer Research.