Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
The Krebs cycle plays such a fundamental role in sustaining life that it is taught in every high school biology course. This metabolic cycle describes a historic moment in the evolution of life on earth – the transition to generating energy with the help of oxygen. In 1953, a Nobel Prize was granted to the Jewish German biochemist Hans Krebs who discovered the circular pathway, also known as the citric acid cycle; its iconic diagram remains a cornerstone of cellular biology. When Dr. Lia Heinemann-Yerushalmi of Prof. Elazar Zelzer’s lab at the Weizmann Institute of Science realized that a new arrow could be added to this diagram, she was awed. “The Krebs cycle is featured in all the biochemistry textbooks. The ability to expand such a classic diagram is truly exciting.”

To better understand the discovery made by Zelzer’s team, we must travel back in time – way back. The first creatures that lived on our planet generated energy by breaking down sugar in a process called glycolysis. This primordial metabolic pathway is not dependent on oxygen, and it is found in creatures that exist today, from bacteria to humans. As life on Earth evolved, so did energy-generating metabolism: The Krebs cycle, which relies on an oxygenated environment, emerged. This newer cycle generates about 18 times more energy than glycolysis, which allows it to support more complex, sophisticated life forms. However, the Krebs cycle did not replace glycolysis as a means of energy generation. Instead, they are both present in advanced life forms, allowing cells to alternate between the two in order to adapt to different situations and environments. Furthermore, certain body tissues and developmental processes require low-oxygen conditions to function, and so rely primarily on glycolysis as an energy source.
""The Krebs cycle is featured in all the biochemistry textbooks. The ability to expand such a classic diagram is truly exciting"
Zelzer’s lab at Weizmann’s Molecular Genetics Department doesn’t normally research metabolic pathways; its usual focus is bone and skeletal development. In fact, their interest in the breakdown of sugars was initially piqued as part of an investigation involving cartilage. Cartilage is among the body tissues that require low-oxygen conditions to develop, relying on glycolysis for energy generation. A team of scientists led by Dr. Lital Bentovim sought to understand why low-oxygen conditions suit cartilage tissue so much better that it has “rejected” the energy advantages of oxygen supply.
To examine this question, the researchers sought to intervene in the process of cellular breathing in mouse embryos, causing their cartilage cells to generate energy via the Krebs cycle instead of glycolysis. To this end, they developed a genetic model of mouse embryos that cannot express pyruvate dehydrogenase kinase, or PDK proteins – a family of enzymes responsible for regulating the switching between metabolic pathways that break down sugar. The results were utterly unexpected: Despite the absence of the enzymes that regulate cellular breathing, the embryos developed normally. The researchers then realized it was no wonder that the embryos developed normally: The regulation of the alternation between the different breathing pathways was preserved in the absence of the proteins considered vital to the process.
In terms of their questions regarding cartilage tissue, this meant that the researchers had hit a dead end. However, Heinemann-Yerushalmi, then a student in Zelzer’s lab, couldn’t stop thinking about the surprising findings and insisted on getting to the bottom of their metabolic source. Her determination led the research team to continue their investigation. That was precisely what led them to make a new discovery in well-trodden territory: The scientists found a backup system in the Krebs cycle. The researchers demonstrated that, in the absence of PDK-family enzymes, an enzyme called branched chain ketoacid dehydrogenase kinase, or BCKDK “steps in.” Until then, BCKDK was known for its participation in another seemingly separate metabolic pathway: the pathway responsible for breaking down branched-chain amino acids.
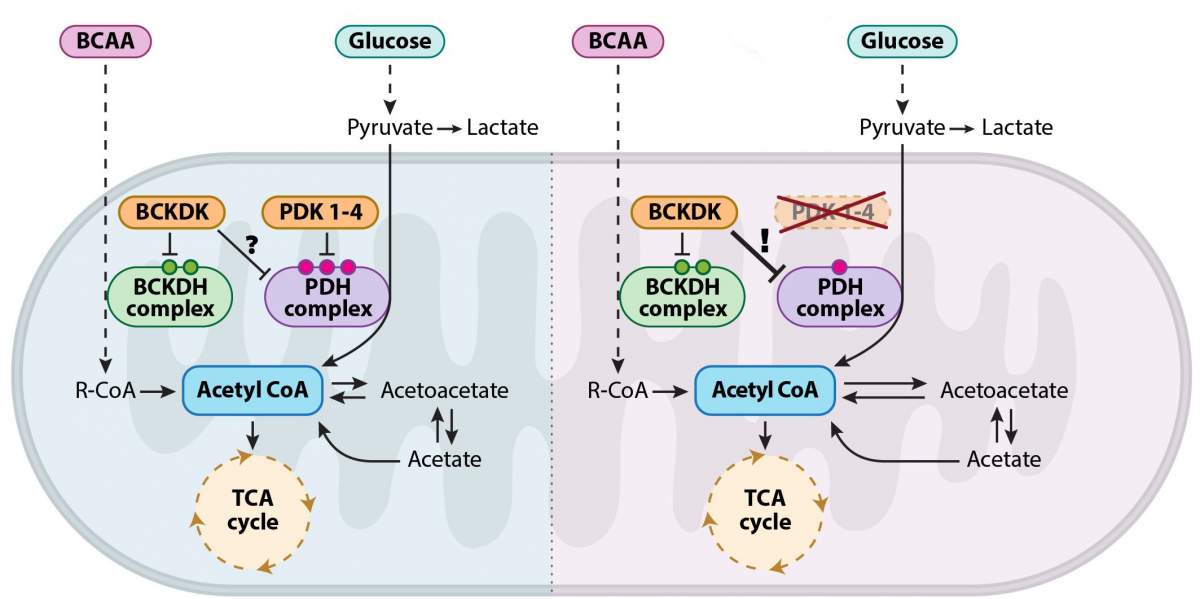
Apart from breaking new ground in basic science, these findings may have implications for the understanding and treatment of many diseases involving changes in the metabolic state of cells. In the past twenty years, studies have shown that PDK enzymes are involved in cancer, in metabolic diseases such as diabetes and in degenerative nerve diseases like Huntington’s. These findings laid the foundation for the development of a variety of new drugs intended to inhibit the activity of PDK enzymes. However, the use of such drugs is limited, owing to low efficacy and the absence of a genetic model that allows a comprehensive physiological assessment of their effect. The new Weizmann research may explain the low efficacy of existing drugs and pave the way for the development of new, more effective treatments for these diseases.
ATP is the “energy currency” of the human body. For each sugar molecule, glycolysis produces 2 molecules of ATP, while the Krebs cycle produces 36. This makes the Krebs cycle about 18 times more effective.
Prof. Zelzer is the Director of the David and Fela Shapell Family Center for Genetic Disorders Research.
Prof. Zelzer’s research is supported by the Estate of Mr. & Mrs. van Adelsbergen.