Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Our family origins tend to shape our future in many ways. A Weizmann Institute of Science study, published today in Nature, found that the same holds true for blood vessels. The researchers discovered blood vessels forming from unexpected progenitors and went on to show that this unusual origin determines the vessels’ future function.
“We found that blood vessels must derive from the right source in order to function properly – it’s as if they remember where they came from,” says team leader Prof. Karina Yaniv.

Blood vessels supplying different organs vary significantly from one to another. For example, because the kidneys engage in filtration, their blood vessel walls have small holes that enable the efficient passage of substances. In the brain, the same walls are nearly hermetic, ensuring a protective blockage known as the blood-brain barrier. Blood vessel walls in the lungs are suited to yet another task, that of facilitating gaseous exchange.
Despite the vital importance of the vascular system, how such differences between various blood vessels come about is still poorly understood. Until now, these vessels were known to originate from two sources – existing blood vessels or progenitor cells that mature and differentiate to form the vessel walls. In the new study, postdoctoral fellow Dr. Rudra N. Das, working in Yaniv’s lab in the Immunology and Regenerative Biology Department, discovered that blood vessels can develop from a previously unknown source: lymphatic vessels. This third sort was revealed in transgenic zebrafish whose cells were labeled with newly established fluorescent markers that enable tracing.
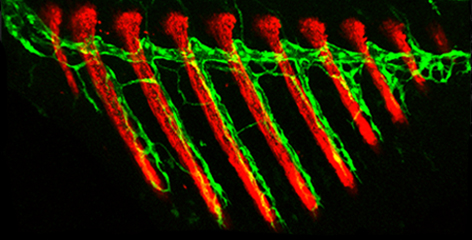
“It was known that blood vessels can give rise to lymphatic vessels, but we’ve shown for the first time that the reverse process can also take place in the course of normal development and growth,” Das says. By tracing the growth of fins on the body of a juvenile zebrafish, Das saw that even before the bones had formed, the first structures to emerge in a fin were lymphatic vessels. Some of these vessels then lost their characteristic features, transforming themselves into blood vessels.
"On a more general level, we’ve demonstrated a link between the ‘biography’ of a blood vessel cell and its function in the adult organism"
This seemed inexplicably wasteful: Why hadn’t the blood vessels in the fins simply sprouted from a large nearby blood vessel? Das and colleagues provided an explanation by analyzing mutant zebrafish that lacked lymphatic vessels. They found that when lymphatic vessels were absent, the blood vessels did sprout in the growing fins of these mutants by branching from existing, nearby blood vessels. Surprisingly however, in this case the fins grew abnormally, with malformed bones and internal bleeding. A comparison revealed that in the mutant fish, excessive numbers of red blood cells entered the newly formed blood vessels in the fins, whereas in regular fish with lymphatic-derived blood vessels, this entry was controlled and restricted.
The scarcity of red blood cells apparently created low-oxygen conditions known to benefit well-ordered bone development. In the mutant fish, on the other hand, an excess of red blood cells disrupted these conditions, which could well explain the observed abnormalities. In other words, only those blood vessels that had matured from lymphatic vessels were perfectly suited to their specialized function – in this case, proper fin development.
Since zebrafish, unlike mammals, exhibit a remarkable capacity for regenerating most of their organs, Das and colleagues set out to explore how a fin would regrow following injury. They saw that the entire process they had observed during the fins’ development repeated itself during its regeneration – namely, lymphatic vessels grew first, and only later did they transform into blood vessels. “This finding supports the idea that creating blood vessels from different cell types is no accident – it serves the body’s needs,” Das says.
The study’s findings are likely to be relevant to vertebrates other than zebrafish, humans included. “In past studies, whatever we discovered in fish was usually shown to be true for mammals as well,” Yaniv says.
She adds: “On a more general level, we’ve demonstrated a link between the ‘biography’ of a blood vessel cell and its function in the adult organism. We’ve shown that a cell’s identity is shaped not only by its place of ‘residence,’ or the kinds of signals it receives from surrounding tissue, but also by the identity of its ‘parents.’”
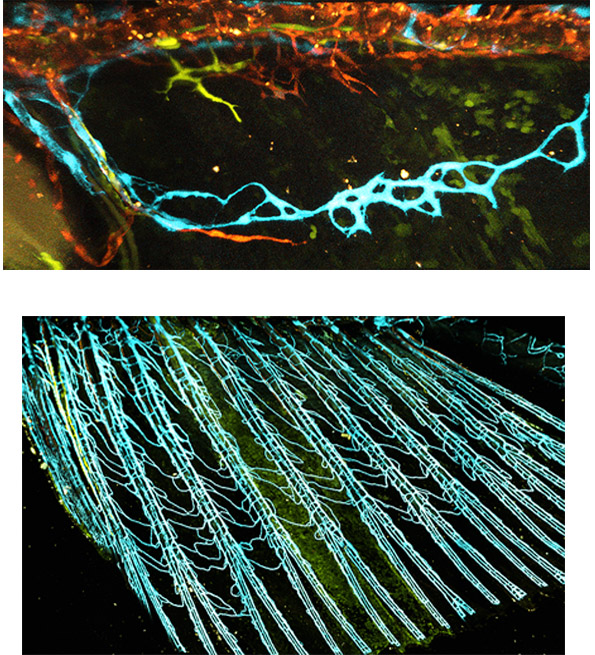
The study could lead to new research paths in medicine and human development studies. It might, for example, help clarify the function of specialized vasculature in the human placenta that enables the establishment of a low-oxygen environment for embryo development. It could also contribute to the fight against common diseases: Heart attacks might be easier to prevent and treat if we identify the special features of the heart’s coronary vessels; new therapies may be developed to starve cancer of its blood supply if we know how exactly this supply comes about; and knowing how the brain’s blood vessels become impermeable may help deliver drugs to brain tissues more effectively. In yet another crucial direction, the findings may have application in tissue engineering, helping supply each tissue with the kind of vessel it needs.
Yaniv, whose lab specializes in studying the lymphatic system, feels particularly vindicated by the new role the study has revealed for lymphatic vessels: “They are usually seen as poor cousins of blood vessels, but perhaps it’s just the opposite. They might actually take precedence in many cases.”
Study participants also included Yaara Tevet, Stav Safriel, Noga Moshe, Giuseppina Lambiase, Dr. Ivan Bassi, Dr. Julian Nicenboim and Dr. Roi Avraham of Weizmann’s Immunology and Regenerative Biology Department; Dr. Yanchao Han and Prof. Kenneth D. Poss of Duke University School of Medicine; Matthias Brückner and Prof. Wiebke Herzog of the Friedrich-Alexander Universität Erlangen-Nürnberg and Max Planck Institute for Molecular Biomedicine; and Dana Hirsch and Dr. Raya Eilam-Altstadter of Weizmann’s Veterinary Resources Department.
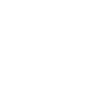
The evolutionary distance between zebrafish and humans is about 400 million years.
Prof. Yaniv’s research is supported by the M. Judith Ruth Institute for Preclinical Brain Research. She is the Director of the Aharon Katzir-Katchalsky Center and the incumbent of the Enid Barden and Aharon J. Jade Professorial Chair in Memory of Canter John Y. Jade.