Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Breaking through concrete walls may not sound like a therapeutic approach, but researchers at the Weizmann Institute of Science suggest that’s just what might help overcome bacterial resistance to antibiotics. In a new study in the journal npj Biofilms and Microbiomes, the researchers propose treating resistant infections by destroying walls that protect colonies of bacteria known as biofilms.
In previous studies, the team of Dr. Ilana Kolodkin-Gal in Weizmann’s Molecular Genetics Department had shown that the bacteria in biofilms build a fortress made of crystalline calcium carbonate, a hard mineral that in nature is the major component of pearls and in the construction industry is used to produce concrete. In the new study, led by Dr. Alona Keren-Paz, the team – including Dr. Vlad Brumfeld of Weizmann’s Chemical Research Support Department and Dr. Yaara Oppenheimer Shaanan, a former post-doctoral fellow in Kolodkin-Gal’s lab – set out to define the structure and function of the biofilm fortress, and to learn how it can be torn down.

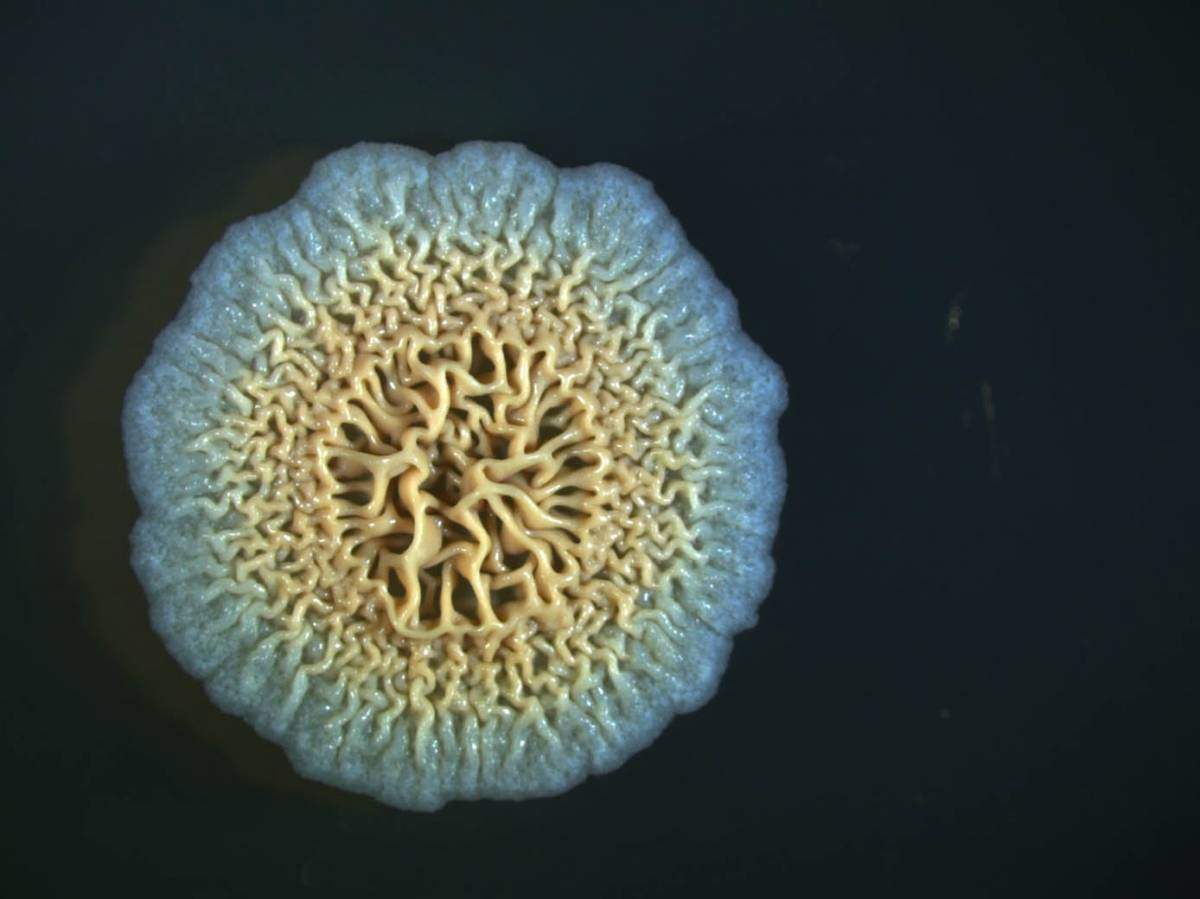
After developing a way of producing three-dimensional images of bacterial biofilms with high-resolution X-ray tomography, the researchers discovered that the fortress image is not just a metaphor. The calcium carbonate, it turned out, forms a well-defined crystalline edifice, complete with walls and a ceiling, around the bacterial colony. “The walls apparently enable the colony to build up thickness, whereas the ceiling protects it from outside influences,” explains Kolodkin-Gal.
The researchers showed that the calcium-carbonate barrier prevents the diffusion of fluorescent dye molecules as well as antibiotics within the bacterial biofilm. This may help explain why biofilms are, on average, a thousand times more resistant to antibiotics than individual bacteria. In fact, in the Weizmann study, individual bacteria within the biofilm were not themselves resistant to antibiotics: When removed from the biofilm, they were killed by an antibiotic drug. The calcium carbonate creates a physical barrier, shielding them from the antibiotic. “Giving an antibiotic in this situation is like throwing it against a wall. It simply won’t get through,” says Kolodkin-Gal.
Giving an antibiotic in this situation is like throwing it against a wall
Bacterial biofilms are known to form on artificial heart valves and other prosthetic devices, and they are believed to cause complications in a variety of chronic diseases, including diabetes, tuberculosis, arthritis and cystic fibrosis. By examining tissue samples obtained from hospitals, the Weizmann scientists have confirmed the presence of bacterial minerals in a number of these diseases.
The researchers found that the calcium carbonate shield makes up about 20 percent of the biofilm’s weight, a percentage similar to that of bones in the human body. Bone indeed resembles the biofilm fortress in many ways: In addition to the use of hard calcium compounds (calcium phosphate in the case of bones), both are held together by a matrix of protein fibers.

The study further revealed that bacteria build their calcium carbonate shield from carbon dioxide and calcium, with the help of an enzyme called urease. Among other things, the enzyme promotes the build-up of the mineral by reducing the acidity of the surrounding environment. When the scientists introduced into the bacterial laboratory dish a small molecule that blocked this enzyme, calcium carbonate failed to form. “Many infections that cannot now be treated by antibiotics due to bacterial resistance may one day become treatable if we manage to destroy the biofilms so that the drug can diffuse throughout,” Kolodkin-Gal says.
In the more immediate future, the findings of the new study may be used in diagnosis to expedite treatment. Imaging can reveal whether the bacteria had formed a biofilm in the body, making it possible to predict whether antibiotics are likely to be effective against the infection.
Growing Buildings with Bacterial Biofilms
A 3D image of the mineral barrier protecting a bacterial biofilm, viewed with high-resolution X-ray tomography
Understanding how calcium carbonate is created in bacterial biofilms may also help achieve the opposite goal: Rather than destroying biofilms, it might enable scientists to find ways of building them up on demand – for example, to design self-healing concrete. Kolodkin-Gal, together with Keren-Paz and scientists from the United Kingdom – architect Prof. Martyn Dade-Robertson of Newcastle University and biologist Dr. Meng Zhang of Northumbria University – recently explored the feasibility of this theoretical scenario in Microbial Biotechnology, in a special issue devoted to the contribution of bacterial biotechnology to sustainable development.
By lacing concrete with bacteria, the scientists suggest, it might be possible to repair cracks or other forms of damage resulting from erosion or land movement. The cracks would be filled by calcium carbonate generated by bacterial biofilms. An extra benefit: The process would make use of carbon dioxide, helping to offset the build-up of this greenhouse gas in the atmosphere.
Dr. Ilana Kolodkin Gal's research is supported by the David and Fela Shapell Family Foundation INCPM Fund for Preclinical Studies; the Helen and Milton A.Kimmelman Center for Biomolecular Structure and Assembly; the Kekst Family Institute for Medical Genetics; David E. and Sheri Stone; and the estate of Helen Nichunsky. Dr. Koldkin-Gal is the incumbent of the Rowland and Sylvia Schaefer Career Development Chair in Perpetuity.