T
he very instant we touch something hot, our hand recoils and we immediately feel pain: The nerve signals informing us of our mistake are transmitted faster than the blink of an eye. These signals zip up and down thin, spiky extensions of the nerve cells that can reach over a meter in length in the human body. To enable the signals to travel rapidly, the nerve cell extensions – the axons – have evolved a unique form: An insulating material called myelin covers the axon in sections, leaving short gaps of exposed nerve cell. The signals jump between these gaps, or nodes, skipping in a fraction of a heartbeat from one end of the axon to the other.
When the axons' myelin sheath is damaged, exposing larger areas of nerve, those signals can get jammed or short-circuited, and the nerve itself may eventually degenerate. A host of neurological diseases, including multiple sclerosis, are tied to myelin malfunction. Prof. Elior Peles, graduate student Ivo Spiegel and their colleagues in the Institute's Molecular Cell Biology Department, as well as fellow scientists in the U.S., have uncovered a mechanism for myelin sheath formation that may point the way toward new therapies for these diseases.
Newly formed axons, when they first extend out from the nerve cell, are not insulated. A specialized set of cells – the glia – serve to install and maintain the myelin sheathing. They revolve around the axons, wrapping them in thin layers of the insulating material with each revolution.
How do the two types of cell coordinate this process? How do the glia know when and where to wrap the myelin? To find out, the scientists first looked for molecules that might act as conduits for messages passed from one cell to the other. They identified four related proteins called Necl (Necl1, 2, 3 and 4) that are found where there is contact between nerve cells. Necl proteins are members of a large family of cell adhesion molecules – proteins and other molecules that sit on the outer membranes of cells and facilitate sticking and communication. Further research narrowed their candidates down to two members of the Necl group. These were Necl1, normally found on the axon surface, and Necl4, which is found on the glial cell membrane. Whether they are intact on the cells' surface or detached and mixed together in the lab, these two recognize each other and stick tightly together.
Like many adhesion molecules, the Necl proteins not only create physical contact between axon and glial cell but also serve to transfer signals to the cell's interior. Signals from axons to glial cells tell them to make the changes needed to undertake myelination. The research team observed what happened when they blocked either of the Necl molecules during myelination in the peripheral nervous system.
They found that production of Necl4 in the glial cells rises when they come into close contact with an unmyelinated axon and when the process of myelination begins. If, however, for some reason, one of these molecules was inactivated or contact between the two blocked, the axons did not myelinate properly, even though they were contacted by glial cells. Often myelin was produced, but the glia did not lay it down on the axon surface in neat layers; instead they created a sort of open, horseshoe-shaped loop that failed to encase the nerve cell in a snug, insulating coat. In the same time period, healthy myelin wrapping was already well under way around most of the axons in the control group.
"What we've discovered is a completely new means of communication between these nervous system cells," says Peles. "The drugs now used to treat multiple sclerosis and other degenerative diseases in which myelin is affected can only slow the disease; they can't stop or cure it. Today, we can't reverse the nerve damage caused by these disorders. But if we could understand the mechanism that controls the process of wrapping the axons in their protective sheath, we might be able to recreate that process in patients."
Prof. Elior Peles's research is supported by the Nella and Leon Benoziyo Center for Neurological Diseases; the J & R Center for Scientific Research; the Kekst Family Center for Medical Genetics; the Dr. Emanuel and Frances Freund Fund for Genomic Modeling; the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; and the Wolgin Prize for Scientific Excellence.
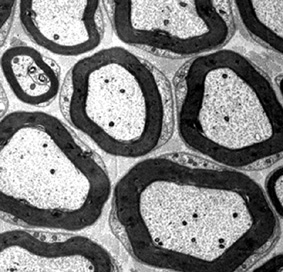
Electron microscope image of a peripheral nerve bundle containing several axons. The black rings are the myelin sheaths
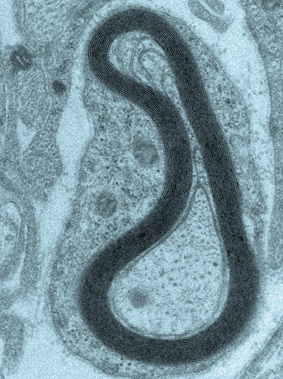
A cross section of a nerve cell as seen under an electron microscope. The forming myelin sheath appears as dark bands surrounding the axon, and the glial cells' cytoplasm can be seen surrounding the sheath


