Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Graphene – a form of carbon that recently garnered its discoverers a Nobel Prize – may one day form the basis of environmentally friendly and efficient electronic devices. Unlike other materials composed of carbon – for example, diamond or graphite – graphene conducts electricity in a useful manner; and as with most other conducting materials, heat is a byproduct that must be figured into any design. A study that recently appeared in Science describes how a highly accurate method of “taking the temperature” of conducting materials revealed a new mechanism by which electrons in graphene give up heat. Understanding this mechanism may help engineers and researchers improve the design of graphene-based materials and devices, as well as providing an intimate glance into a novel microscopic process that has been unexplored until now.
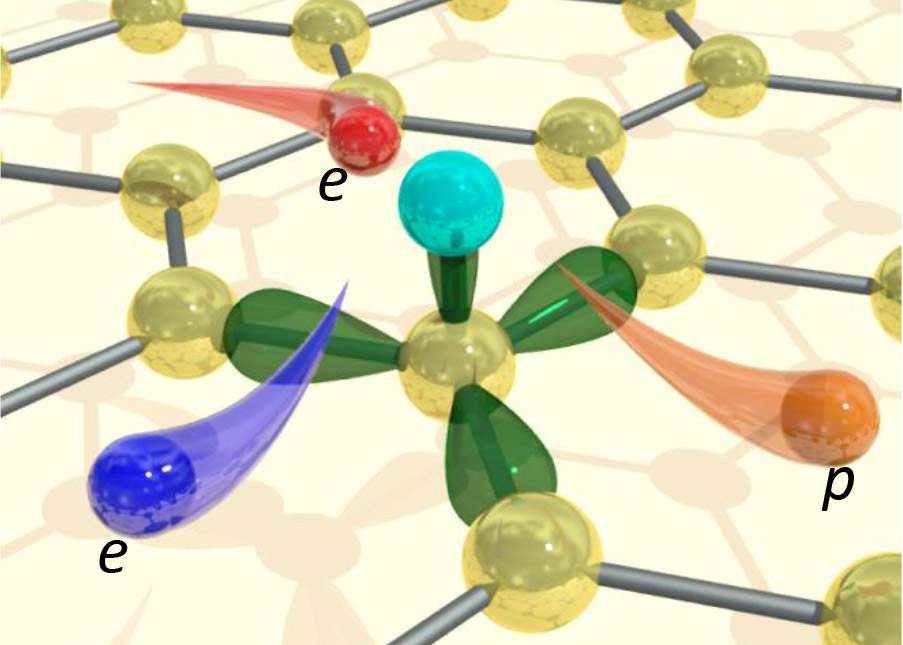
“Graphene is a two-dimensional lattice of carbon atoms that looks like a honeycomb,” explains Dr. Dorri Halbertal, currently a postdoctoral fellow in the lab of Prof. Eli Zeldov of the Weizmann Institute of Science’s Condensed Matter Physics Department. ”For an electron passing through, there is nothing to ‘catch its attention’ – nothing to get it to release its excess energy.” Yet heat clearly does get produced, and for ultra-small devices even a small amount of heating can be a serious matter. How is heat produced in such “ideal” materials as graphene?

To answer the question on the atomic scale, Halbertal and Zeldov teamed up with Dr. Moshe Ben Shalom and Prof. Andre Geim – one of graphene’s discoverers – of the University of Manchester, who provided samples of ultra-pure graphene sandwiched between two thin layers of an inert material. Zeldov and his group made use of a unique measurement system developed in his lab, which they named SQUID on a tip thermometer (SQUID stands for Superconducting Quantum Interference Device). Sitting in an industrial freezer-sized box containing around two tons of concrete, supported by hydraulic lifts and surrounded by a container of helium cooled to around 4 degrees Kelvin – all to isolate the device from the slightest outside disruption – the main part of the SQUID apparatus is a fine quartz needle coated with lead – a metal that upon cooling down turns into a superconductor. The ring at the tip of this needle, just a few tens of nanometers across, is the key to the abilities of this device. It measures the properties of a material by probing – coming as close as possible to the surface without touching it.
This provides a system of measurement that is an improvement on other leading methods by a factor of ten thousand
The SQUID apparatus had originally been designed for measuring tiny disturbances in magnetic fields in materials, but Zeldov and Halbertal realized that it was highly sensitive to even the slightest changes in temperature, and they turned this sensitivity to their advantage. In previous research the team had demonstrated the device’s capabilities, measuring temperature changes in the microkelvin range (the Kelvin scale begins counting from absolute zero – as cold as it can go). This provides a system of measurement that is an improvement on other leading methods by a factor of ten thousand. The unique measurement scheme also enabled the researchers to turn the dissipation on and off at specific sites – that is, the researchers could not only measure the heating at the atomic scale, but control some aspects of it.
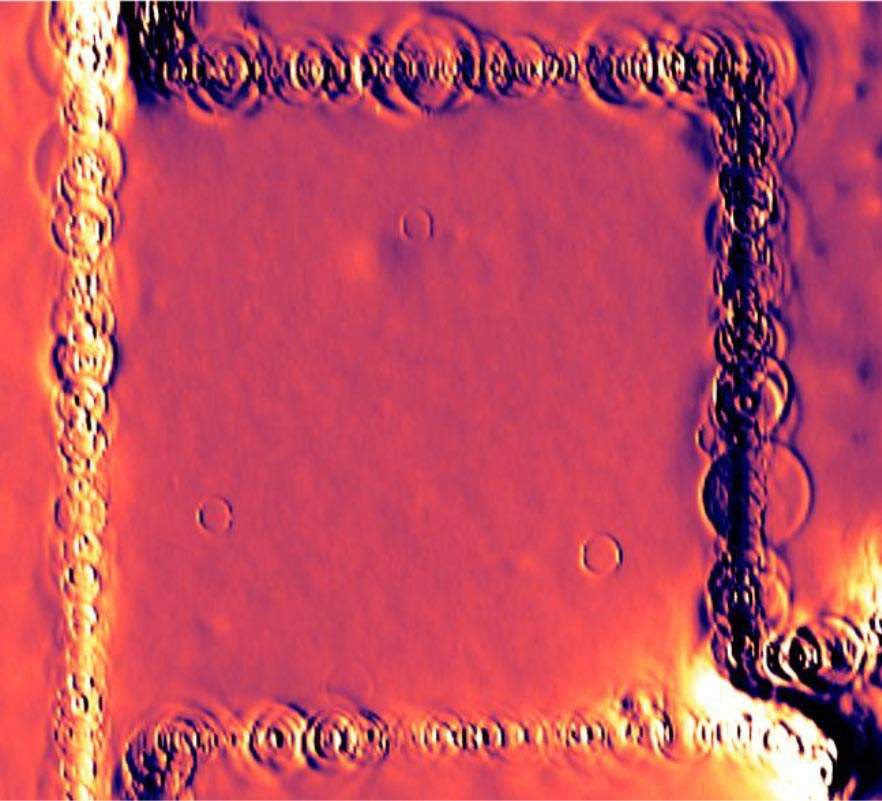
In the present experiment, the researchers found that the electrons released heat at only a handful of localized sites within the body of the sample, but also at quite a few points along the edges. This gave the team the possibility of defining the exact locations at which heat was being generated. “These sites are atomic defects, probably hydrogen atoms that attached themselves to the carbon lattice,” says Halbertal. “The defects are like traps: They can hold the electrons in them for some time before these escape, minus the tiny bit of energy that they release as heat.”
In the images obtained in the experiments, the locations of these atomic defects appeared surrounded by rings. An analysis of these rings – tiny “hot” peaks of energy release – conducted together with Prof. Leonid Levitov of the Massachusetts Institute of Technology, revealed a novel mechanism for the heat release that had not been considered before. This mechanism involves a very specific energy state that the electron requires in order to let go of heat.
The insights provided by this study may be useful to physicists, materials scientists and engineers who work with graphene. But, says Halbertal, because its resolution is so much finer than any other, the method of measurement may in the future have much more to tell us about the basic physics of heat release. “Many processes across scientific disciplines, from biology to supernovae, involve the exchange and conversion of energy,” says Zeldov. “With this method, we can understand how energy dissipation occurs at the level of single electrons.”
Prof. Eli Zeldov's research is supported by the Willner Family Leadership Institute for the Weizmann Institute of Science; the Leona M. and Harry B. Helmsley Charitable Trust; the Gruber Center for Quantum Electronics, which he heads; the Joseph H. and Belle R. Braun Center for Submicron Research, which he heads; the European Research Council; and the Rosa and Emilio Segre Research Award. Prof. Zeldov is the incumbent of the David and Inez Myers Professorial Chair.