Bacterial cells do not wake up one morning and decide to become parents. But there is a point in their cell cycle – after growing sufficiently and replicating their genomes – when they split in two, creating new cells that then repeat the process. What tells the bacterium that it’s time to split apart? Scientists have been divided on the subject.
In the past few years, when trying to uncover the biological signals that determine when a bacterium will divide, scientists have relied on a recently developed technology that allows them to track the life cycles of a single bacterial cell and all of its offspring for hundreds of generations. The problem is that experiments using this system produced contradictory models, each attributing the timing of cell division to a different factor. In a new study, published in Proceedings of the National Academy of Sciences (PNAS), a team headed by Weizmann Institute of Science researchers used statistical reasoning to reveal which cell division model is the most plausible. This method has the advantage of going straight to the issue at stake – identifying the division’s causal factor – without having to expose the exact biological mechanisms involved.

In previous research, scientists tracking the division of bacterial cells had observed that the cells grow by exactly the same volume from the moment of DNA duplication to the cell’s division. From this, they deduced that the processes telling the cell that it should split in two begin during the stage when genetic material is being duplicated. However, studies also showed that the cells grow by exactly the same volume from the moment of their birth to the moment they split. This observation led to the development of a contradictory model, in which the countdown to cell division begins at the cell’s birth. According to this approach, a regulatory protein starts to build up in the cells from the beginning, setting a sort of ticking alarm clock to go off when this protein’s levels reach a certain point. To resolve this contradiction, a third model combined the two, proposing the involvement of both a regulatory protein and genetic replication.
These models were all based on correlation: Scientists noted that growth limits in certain parts of the bacterial cells’ cycle seemed to be synchronous with cell division. But, as any first-year science major can tell you, correlation is not causation. That is, protein accumulation or DNA replication could be happening at the same time as the signal to split, while having no causal relationship with that signal.
In order to decide between the two models of cell division, Prof. Ariel Amir from Weizmann’s Physics of Complex Systems Department and an international team of scientists used conditional independence testing, a statistical tool developed by Judea Pearl, the Israeli-American scientist who was awarded the Turing Prize for his work on the techniques involved in this approach. The researchers applied this testing to data that had been collected, in collaboration with the University of Tennessee, from the growth of hundreds of different E. coli bacteria in different batches. Some of the cells were given conditions that allowed them to divide quickly, while others were grown in conditions that dictated slower growth. The data included the timing of the different stages in the cells’ life cycle, as well as the size of the cells at each stage.
Conditional independence tests pose “if-then” questions that can reveal which correlation is just that – a coincidence that does not involve causation. In work led by Prathitha Kar, a research student at Harvard, the team compared groups of bacterial cells that were of similar size during their DNA duplication stage, but had been different sizes at birth. If the model that claims the timing of cell division depends solely on DNA duplication is correct, then cells of a similar size at duplication would divide at a similar time – irrespective of their size at birth. If the model is wrong, however, and it’s the accumulation of protein from birth that determines when the cells will divide, then cells of dissimilar size at birth would divide at different times, and there would be a correlation between size at birth and size at the time of division.
""The ability to characterize the growth of a wide range of pathogens could pave the way for the development of more effective antibiotics"
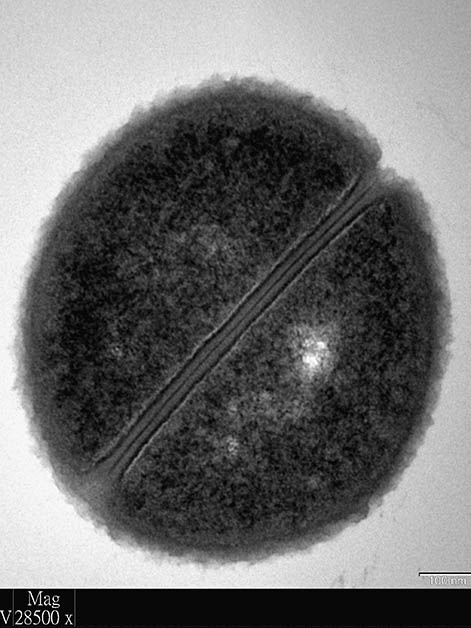
The researchers were surprised to discover that both models are valid, but not in the way the third “hybrid” model had proposed. When the bacterial cells grew at a slow pace, it was the DNA duplication processes alone that determined when a cell would divide. When the cells’ growth rate was faster, however, the situation became more complex, and researchers discovered that both the processes that began at birth and the DNA duplication combined to determine when the cell would split. Finally, the researchers revealed a visual clue that cell division had reached a point of no return: A cell is destined to split into two cells the moment that a ring starts to form at its center.
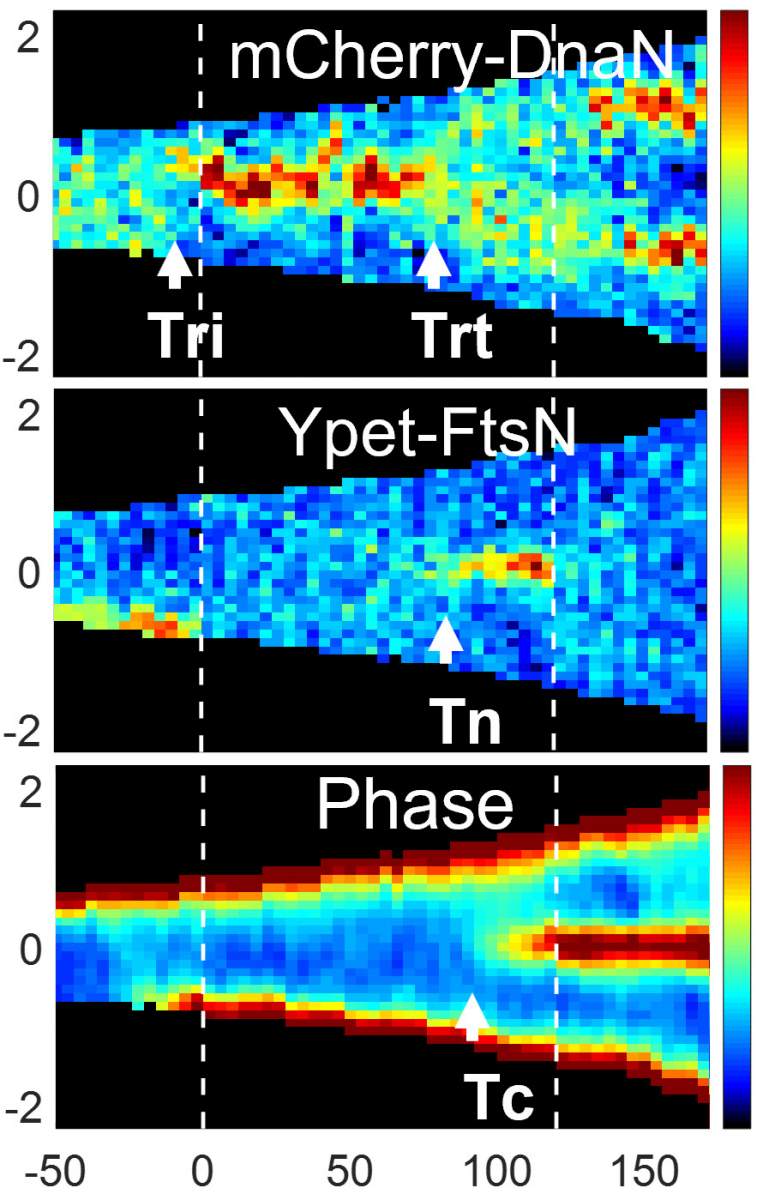
The scientists also used conditional independence testing to disprove a commonly held theory that the timing of DNA duplication in one generation rigidly determines this timing in the next. Rather, they showed that processes occurring in the parent cell after its DNA duplication had already begun can affect when the daughter cells begin to replicate their own genetic material.
“Using statistical methods to confirm a causal relationship affords us a better understanding of the processes of growth and division in bacterial cells,” says Amir. “Conditional independence tests have been commonly used in fields like epidemiology, economics and so on. I believe that the ability to characterize the growth and reproduction processes of a wide range of pathogens will pave the way for the development of more effective antibiotics in the future.”
Also participating in the study were Dr. Sriram Tiruvadi-Krishnan, Prof. Jaana Männik and Prof. Jaan Männik from the University of Tennessee.