Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
More than a hundred years ago, scientists looking through microscopes at red blood cells noticed that the cells’ membranes were vibrating rapidly. They termed this motion “flickering.” The causes of this flickering have now been clarified in a new study published in Nature Physics, in which the Weizmann Institute’s Prof. Nir Gov collaborated with German and French scientists. The findings promise to shed new light on the motion of various cells in the healthy body and in numerous disease processes.

Historically, opinions on flickering were sharply divided. Some scientists thought it was a passive process, resulting from the random motion that normally occurs in molecules as they gain or lose heat. Others argued that flickering resulted from active, energy-consuming processes in the cell.
More than a decade ago, when Gov, now a professor in the Weizmann Institute’s Chemical Physics Department, was a postdoctoral fellow at Weizmann, he built a theoretical model that supported the latter hypothesis. It described flickering as an active process involving ATP, the molecule that cells use for energy transfer. In Gov’s model metabolic processes fueled by ATP produce chemical changes that cause the cells’ supporting structure, called the cytoskeleton, to become temporarily disconnected from part of the cellular membrane. Each time this happens, this portion of the membrane becomes slack and expands. When the connection is reestablished, the membrane tightens up and contracts again.
In the new study, Dr. Timo Betz, of the University of Münster, and his colleagues built a remarkable installation that enabled them to examine the mechanisms of flickering with unprecedented precision. In this installation, a single red blood cell – measuring six to eight microns (thousandths of a millimeter) across – is suspended in solution by four tiny silicon beads, three of which hold the cell in place while the fourth serves as a probe to track the cell’s motion. By manipulating the beads with thin laser beams known as optical tweezers, the scientists could detect the cell’s free fluctuations or gently pull on the cell in order to measure its mechanical properties.
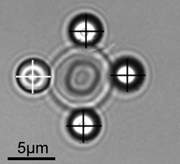
Amazingly, both explanations for flickering – both a passive and an active mechanism – proved to be right, but different mechanisms prevailed, depending on the timescale involved. During time intervals lasting less than a tenth of a second, the flickering is mainly due to passive, temperature-induced motion. But at longer time intervals the cell’s fluctuations were greater than could be expected from thermal motion alone. In fact the measurements at these intervals fit the predictions made by Gov’s model, suggesting that much of the flickering is a byproduct of energy consumption by the cell during its constant remodeling of the cytoskeleton. Computer simulations mimicking the exact shape of red blood cells further supported these conclusions.
The study may help biologists investigate whether flickering affects the functioning of red blood cells. Reduced flickering, for example, may be a sign of cell rigidity, which in turn can interfere with the flow of these cells through narrow blood vessels. If the degree of flickering is indeed shown to be altered by disease, its measurement may become part of the diagnosis in disorders such as stroke, heart disease or diabetes, whose symptoms result, at least in part, from disruptions in blood flow. In the future, flickering may also prove relevant to understanding various forms of motion in other cells of the body – for example, immune cells moving to the site of infection, muscle cells contracting and the division of cells in the body.
Taking part in this study were Dr. Hervé Turlier, of the European Molecular Biology Laboratory in Heidelberg, Germany; Dr. Dmitry Fedosov, Dr. Thosten Auth and Prof. Gerhard Gompper, from Forschungszentrum Jülich; Dr. Basile Audoly, from the University of Paris; and Dr. Cécile Sykes and Prof. Jean-François Joanny, of the Institut Curie in Paris.
Prof. Nir Gov's research is supported by the Yeda-Sela Center for Basic Research; and the Clore Center for Biological Physics. Prof. Gov is the incumbent of the Lee and William Abramowitz Professorial Chair of Biophysics.