Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Prof. Sima Lev is aiming to defeat a particularly treacherous type of breast cancer. Cancer is never good news, but the type of malignant breast tumor known as triple negative is harder to treat than others. It is so called because it lacks the three receptors that in other breast cancer types serve as targets for anti-cancer drugs. Thus the treatment options are very limited, and even when chemotherapy does work, the cancer often recurs. Triple negative breast cancer spreads throughout the body so aggressively that even when it is discovered in a very early stage, it has often already stolen into distant body organs, ultimately causing metastasis. About one-fifth of all breast cancers are triple negative, which means that more than 300,000 women worldwide are diagnosed every year with this form of malignancy.
In a new study published in Nature Communications, Lev and postdoctoral fellow Dr. Nandini Verma, working in the Weizmann Institute’s Molecular Cell Biology Department, have provided new insight into triple negative breast cancer that in the future may help treat this malignancy. The scientists focused on the transition that all cancer cells, including triple negative breast cancer cells, must undergo in order to metastasize. This is a transition from a relatively steady state, characterized by effective contact with adjacent cells and the surrounding matrix, to a more invasive one, in which the cancer cells become mobile, assume a spindle-like shape and lose the structural elements that once enabled them to stick to their surroundings.
The scientists have identified a molecular switch, a signaling enzyme called PYK2 that plays a key role in this cellular transition to an invasive, pro-metastatic state. When Lev discovered this enzyme some two decades ago, she found that it conveys signals leading to cellular growth, division and mobility. These signals are essential when cells grow, for example, in the embryo or in the course of wound healing. In the new study, Verma and other members of Lev’s team, Omer Keinan and Michael Selitrennik, revealed how PYK2 enhances breast cancer metastasis.
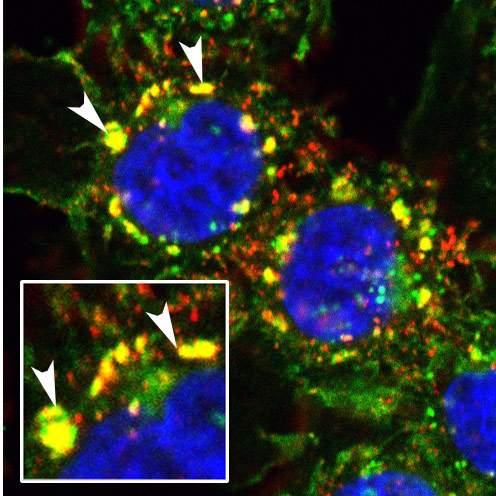
The team at first established that PYK2 promotes the transition toward invasiveness by exaggerating the usual signals it transfers under healthy growth conditions. Next, the scientists discovered that in triple negative breast cancer, two entirely different mechanisms cause the normally short signal transmitted by PYK2 to become a sort of "stuck doorbell."
PYK2 literally causes the signal inducing the transition toward invasiveness to remain in the “on” position for a long time. It achieves that by attenuating the “off” switch that normally terminates the signal. On top of this, PYK2 participates in a positive feedback loop: In other words, the signal it transmits not only enhances the pro-metastatic transition but also promotes its own production, so that PYK2 levels in the cell keep increasing.
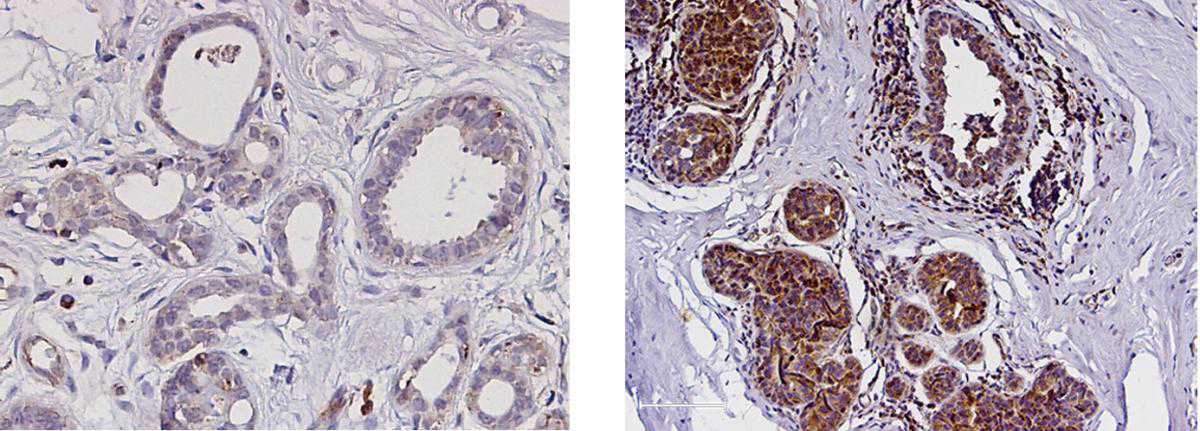
Working with more than 90 tissue samples from breast cancer patients, the scientists found supportive evidence for the central role played by PYK2 in the progression and spread of this malignancy. The more aggressive tumors were shown to contain higher PYK2 levels. Moreover, cancers that had spread to the lymph nodes were found to have higher PYK2 levels than those that had not. When the scientists manipulated the cells to eliminate PYK2, the invasive cells reverted to their normal state – a finding suggesting that PYK2 is not just a simply relay, but that it works as a master switch enabling the pro-metastatic transition.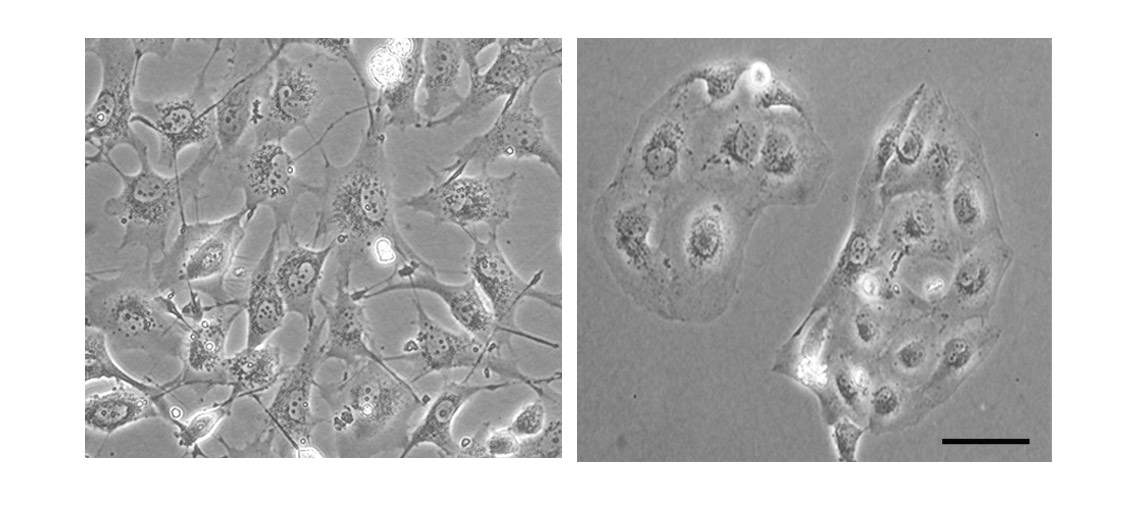
In light of these findings, PYK2 may in the future serve as a target for a new therapy for triple negative breast cancer. Small molecules can be developed to block the PYK2 signal, and they can be used as drugs to be administered in combination with other therapies aimed at this cancer.
Prof. Sima Lev's research is supported by the Jeanne and Joseph Nissim Foundation for Life Sciences Research; the Maurice and Vivienne Wohl Biology Endowment; the De Benedetti Foundation-Cherasco 1547; the Rising Tide Foundation; Luis and Miriam Stillmann, Mexico; and the estate of Georges Lustgarten. Prof. Lev is the incumbent of the Joyce and Ben B. Eisenberg Professorial Chair of Molecular Endocrinology and Cancer Research.