Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

By the time a fertilized egg completes the trip from ovaries to uterus, it has already been transformed into a tiny ball of cells called a blastocyst. Its next step is fraught with risks: Around half of all blastocysts will fail in their attempts to implant in the uterus. Some will be rejected due to abnormalities, but others won't make it because the uterus for some reason doesn't provide them with the conditions they need to thrive and grow.
From the moment a blastocyst settles on an inviting spot on the uterus lining, the surrounding cells begin to change and grow. Soon, a spongy, blood-rich mass called the decidua forms from the uterine tissue. Most of the decidua disappears once a proper placenta is formed, but the decidua tissue is crucial for nurturing the new embryo during the precarious first stage of pregnancy.
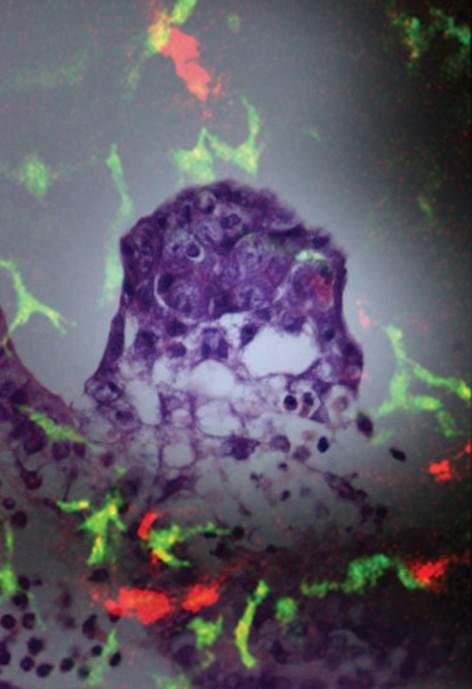
Changes in the uterine wall at this time include alterations in the population of immune cells that normally inhabit the uterus. For instance, cells called dendritic cells cluster near the newly implanted blastocyst. Dendritic cells of different types are found throughout the body, where they generally help to form one of its first lines of defense against invading microorganisms, scouting the tissues and alerting the immune system to potential threats.
So what are these immune system fighters doing in the vicinity of the newly implanted blastocyst? The prevailing hypothesis is that these cells somehow play an opposite, protective role in implantation. Uterine dendritic cells, so the theory goes, help to prevent other immune cells from attacking the tiny blastocyst, which shares only half of its genes with its mother and might therefore be regarded by her immune system as a foreign menace.
To test this theory, two research students, Tal Birnberg and Vicki Plaks from the laboratories of Dr. Steffen Jung at the Institute's Immunology Department, and Profs. Nava Dekel and Michal Neeman of the Biological Regulation Department, in collaboration with Dr. Gil Mor of the Yale University School of Medicine, cross-bred mice so that the embryos were genetically identical to the mothers. They then removed the dendritic cells from the uterus using an in vivo cell depletion model developed by Jung during his postdoctoral studies at New York University. Because the immune system could not identify the embryo as foreign, there was no cause for rejection of the blastocysts – but implantation failed anyway.
If they don't defend the blastocysts, what role do the dendritic cells play? To investigate, the scientists again depleted the uterus of dendritic cells, this time in mice that had been induced to develop a decidua without a blastocyst (a technique perfected by Dekel's team). In every case, in vivo MRI studies showed the decidua-forming cells multiplied more slowly and didn't differentiate properly, and new blood vessels were slow to grow.
These findings led the researchers to the somewhat startling conclusion that these particular dendritic cells had taken on a completely new function. Rather than acting as front-line warriors of the immune system, or even protectors of the new embryo, they seemed to be involved in remodeling the nursery, helping to reshape the tissue surrounding the implantation site to provide for the needs of the new embryo. The scientists hope to continue this line of research and to identify exactly what factors are involved in creating a viable decidua. As well as shedding light on this vital but little understood stage of pregnancy, future studies based on this research may help to advance treatments for infertility.
Prof. Nava Dekel's research is support-ed by the Dwek Family Biomedical Research Fund; the Kirk Center for Childhood Cancer and Immunological Disorders; and the Dr. Pearl H. Levine Foundation for Research in the Neuro-sciences. Prof. Dekel is the incumbent of the Philip M. Klutznick Professorial Chair of Developmental Biology.
Dr. Steffen Jung's research is supported by the Kekst Family Center for Medical Genetics; the Kirk Center for Childhood Cancer and Immunological Disorders; the Swiss Society of Friends of the Weizmann Institute of Science; the Fritz Thyssen Stiftung; the estate of Edith Goldensohn; the Women's Health Research Center funded by: the Bennett-Pritzker Endowment Fund, the Marvelle Koffler Program for Breast Cancer Research, the Harry and Jeanette Weinberg Women's Health Research Endowment and the Oprah Winfrey Biomedical Research Fund; and the Center for Health Sciences funded by the Dwek Family Biomedical Research Fund and the Maria and Bernhard Zondek Hormone Research Fund. Dr. Jung is the incumbent of the Pauline Recanati Career Development Chair of Immunology.
Prof. Michal Neeman's research is supported by the Clore Center for Biological Physics; the Abisch Frenkel Foundation for the Promotion of Life Sciences; the Phyllis and Joseph Gurwin Fund for Scientific Advancement; the Ridgefield Foundation; the Women's Health Research Center funded by: the Bennett-Pritzker Endowment Fund, the Marvelle Koffler Program for Breast Cancer Research, the Harry and Jeanette Weinberg Women's Health Research Endowment and the Oprah Winfrey Biomedical Research Fund. Prof. Neeman is the incumbent of the Helen and Morris Mauerberger Chair in Biological Sciences.