Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

Proteins are dynamic entities – given to internal motion, as well as constantly exchanging loosely bound hydrogen atoms with surrounding water. Conventional wisdom would state that these atomic exchanges occur more readily when proteins are unfolded, since folding into compact, three-dimensional structures closes off the water’s access to sections of the protein. In fact, hydrogen exchange rates have even served as a measure for protein folding propensity – an important aspect of structure that deeply affects protein function. Now Weizmann Institute of Science researchers, using a new spectroscopic tool based on hyperpolarized nuclear magnetic resonance (NMR), have shown that it’s often useful to challenge conventional wisdom.
In the new study, two Weizmann research groups, led by Prof. Lucio Frydman of the Chemical and Biological Physics Department and Dr. Rina Rosenzweig of the Structural Biology Department, collaborated to experimentally examine the back-and-forth exchange of hydrogen atoms between proteins and their aqueous environment. Protein folding is a central topic in Rosenzweig’s lab, including investigating supportive proteins that, among other functions, facilitate the folding of others. The scientists applied a “hyper-water” method developed by Frydman and his coworkers for vastly increasing the sensitivity of certain biomolecular NMR experiments. It consists of freezing water mixed with a small amount of a stable radical to a temperature close to absolute zero, which under suitable conditions “hyperpolarizes” the hydrogen nuclei – that is, aligns them with the external magnetic field to a much higher degree than normal. This hyperpolarized ice is then instantly melted and poured onto the biomolecules of interest – in this case, proteins. By exchanging its own loosely-bound hydrogen atoms with those within the protein, the hyperpolarized water then enables the NMR to pick up, for each of the protein’s amino acids, signals that are several-fold stronger than usual for a protein. The method thus makes it possible to study the interactions between water and proteins at an unprecedented level of detail.

Dr. Or Szekely – then a graduate student in Frydman’s lab and later a postdoctoral fellow in Rosenzweig’s lab – together with Mihajlo Novakovic and Dr. Gregory Olsen, applied this method to four proteins, each characterized by a different folding pattern. The first one had a well-folded structure, while the second one was entirely unfolded. The structures of the third and fourth proteins existed in a dynamic equilibrium, constantly switching between folded and unfolded states, and doing so at rates that the researchers could control by changing the sample temperature. In the first of these “mixed” proteins, the folding-unfolding occurred relatively slowly, at a rate of approximately once per second. In contrast, in the second of the “mixed” proteins, this was quite fast, occurring 20 -30 times per second.
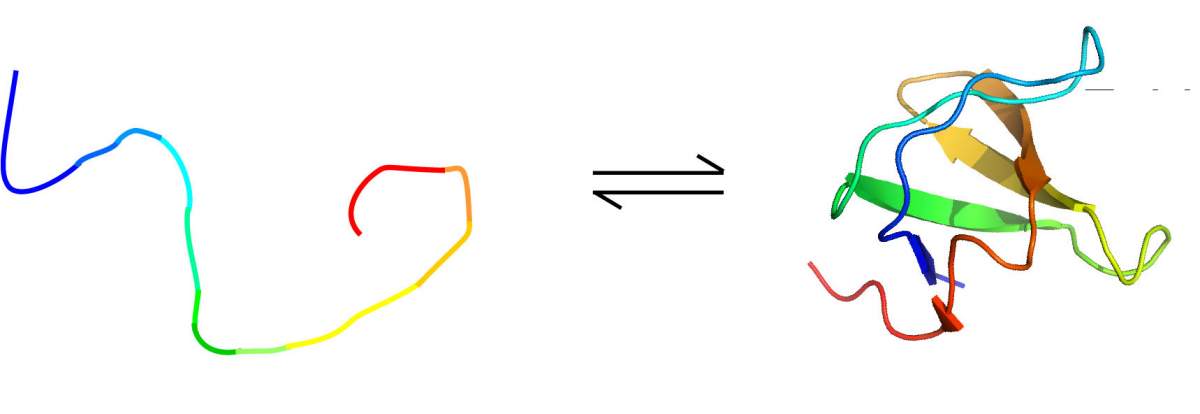
In the first three proteins, the NMR findings fit in with the conventional wisdom: As expected, the “hyper-water” enabled the NMR to pick up much more powerfully enhanced signals from the exchanged hydrogen atoms in the unfolded proteins or unstructured sections than in folded ones. In the slowly interconverting protein, for instance, the NMR protein signals in the unfolded state were enhanced about ten times more than those in the folded one. The fourth protein, though – the one in which the folded and unfolded regions switched rapidly – served up a major surprise: Here the signal enhancements were significantly greater – often by a factor of three or more – in the folded protein segments than in their unfolded counterparts.

“We were so surprised that we repeated the experiments over and over for several months, to make sure we hadn’t made a mistake,” Frydman says.
The scientists have proposed several theoretical explanations for the surprising results and are planning further experiments to determine which one of these is true. Their findings, though, already suggest that the existing paradigm needs to be reexamined: Folding cannot automatically be assumed to reflect a protein’s accessibility to the surrounding water.
Prof. Lucio Frydman is Head of the Clore Institute for High-Field Magnetic Resonance Imaging and Spectroscopy; and Head of the Fritz Haber Center for Physical Chemistry. His research is also supported by the Leona M. and Harry B. Helmsley Charitable Trust; the Dr. Dvora and Haim Teitelbaum Endowment Fund; the Ben B. and Joyce E. Eisenberg Foundation; and the Harold Perlman Family. Prof. Frydman is the incumbent of the Bertha and Isadore Gudelsky Professorial Chair.
Dr. Rina Rosenzweig's research is supported by the Abisch Frenkel Foundation for the Promotion of Life Sciences; the Blythe Brenden-Mann New Scientist Fund; and the European Research Council.