Weizmann Institute of Science researchers were thrilled when they managed to observe the birth of a protein made by a single DNA molecule: No one had previously seen this event outside a cell. That excitement, however, was only the beginning. The insights from the observation enabled the scientists to devise the smallest-ever, yet self-sufficient, genetic circuit – a feat that in the future might help design artificial cells and create improved nanodevices for use in biotechnology.

“We revealed a really clever genetic design principle in nature, and that’s what enabled us to build a circuit on an individual DNA molecule,” says postdoctoral fellow Dr. Ferdinand Greiss, who led this research in Prof. Roy Bar-Ziv’s lab in Weizmann’s Chemical and Biological Physics Department.
""We’ve shown that the DNA can be used as a miniature hub of activity – when what’s encoded in the DNA stays on the DNA"
Much like an electric circuit, whose components work together to produce a physical effect, such as lighting a bulb, a genetic circuit is a network of cellular components – genes, promoters, regulatory proteins – that together give rise to the expression of genes or another biological process. And just as an electric circuit is an independent unit that can be part of more complex devices, so a genetic circuit is a stand-alone entity that can serve as the basis for artificial biological machines to be used in a variety of medical or biotechnological applications.
Evolution’s amazing accomplishment
The study began with an experiment that produced a most unlikely result. Greiss was studying various aspects of so-called artificial cells, which are the central theme of research in Bar-Ziv’s lab. In one experiment, he was tracing the process by which genes are expressed – that is, by which they produce the proteins they encode – in a genetic circuit. He engineered DNA from E. coli bacteria to create a circuit that contained a gene encoding a regulatory protein intended to act as an “on” switch for another gene. He also added a fluorescent tag, which was supposed to light up when that other gene was expressed.
Greiss started out by integrating only about ten of the engineered DNA molecules into an artificial cell, which he then filled with a solution mimicking the inside of a cellular compartment. Normally, for gene circuits to work in either living or artificial cells, the DNA has to generate hundreds to thousands of copies of regulatory proteins; these float about the cell environment until ultimately meeting up with their intended DNA segments, regulating their expression. This was not supposed to happen so easily when only a handful of regulatory proteins were drifting like bits of flotsam in a sea of solution in Greiss’s experiment. Yet much to the researchers’ surprise, the fluorescent tag soon announced that their gene of interest, switched on by the regulatory proteins, had undergone expression. How did so few regulatory proteins find their way to the DNA in the artificial cell so quickly?
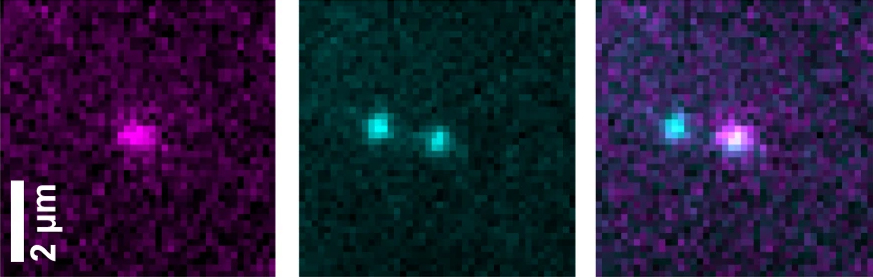
Even more surprising, when Greiss attached a single DNA molecule to a surface and immersed this surface in a solution, the fluorescent tag still lit up very soon. In other words, even at the most extreme dilution possible, the “on” switch protein had somehow connected with its DNA target at record speed.
The only plausible explanation was that after the regulatory protein was manufactured, it remained temporarily attached to the DNA. In fact, studies by labs elsewhere had in the past suggested that this was the case in E. coli, but proving the existence of such tethering was impossible with existing technologies, in part because protein synthesis works within less than a minute, while standard fluorescent tags take several minutes to light up.
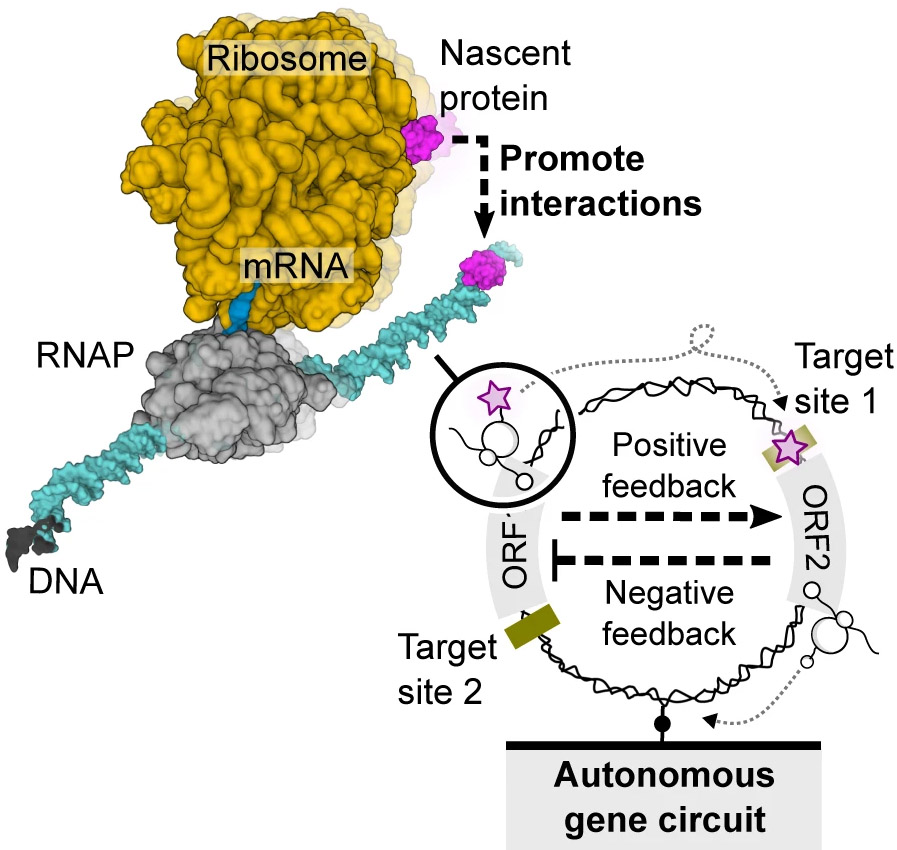
In collaboration with scientists from Germany, Greiss developed a new kind of tag that lights up within dozens of seconds, much faster than the usual fluorescent labels. He then spent several months building an installation that would allow him to use this tag for observing individual molecules under the microscope. Thus, he and colleagues were finally able to directly observe, for the first time, how a newly made regulatory protein indeed lingered on the E. coli DNA, as if tethered by an umbilical cord until gene expression was complete. Using the same setup, the researchers were then able to observe how that single DNA molecule gave birth to a protein encoded by their gene of interest.
“It was fascinating for me to discover how evolution has managed to accomplish such economical design,” Greiss says, adding that the tethering they had observed experimentally confirmed the existence of a genetic design principle that until then had only been identified in theory.
The researchers then employed this design principle to wire up a full genetic circuit on a single DNA molecule. Apart from the “on” switch, they engineered the molecule to contain an “off” switch. Both switches were programmed to remain tethered to the DNA until their job was done. In the future, numerous functions could be programmed into nanocircuits built on the same principle.
“We’ve shown that the DNA can be used as a miniature hub of activity – when what’s encoded in the DNA stays on the DNA,” Bar-Ziv says. “This means that we can think of a single DNA molecule as a self-sustained entity – it doesn’t need to be placed into its own enclosure; rather, it can work in a compartment of any volume. It can therefore serve as an independent nanodevice for a variety of future applications, from biocomputing to medical diagnostics and therapy.”