“At first sight, it's a mix of methods that couldn't possibly work together, and yet we have found it to work very well,” says
Prof. Michael Elbaum of the Weizmann Institute’s Materials and Interfaces Department. Together with Dr. Sharon Grayer Wolf of Weizmann and their colleague Dr. Lothar Houben from the Ernst Ruska Center in Juelich, Germany, the team has developed a new approach to imaging biological samples in three dimensions using an electron microscope.
An electron microscope is a powerful tool able to image specimens at extraordinarily high magnification and resolution, allowing scientists to see cells, molecules and sometimes even atoms. But the microscope’s harsh environment – high vacuum and merciless bombardment with electrons – is hard on biological specimens, damaging and distorting their delicate structures. Added to this, the sample must scatter electrons – without absorbing their energy – in order to form a high-contrast image. Because biological samples are not very good at scattering electrons, a classic approach is to stain organic structures with atoms of heavy metals. This enhances contrast in the images, but the preparations can cause additional harm to the already-delicate specimen. A newer method involves the ultra-rapid cooling of biological specimens. The cooling is so fast that the surrounding water instantly solidifies into “glass” without crystallizing and so preserves proteins and cells in true-to-life form. This protects them against the vacuum, but the problem of weak scattering remains an issue. In addition, in 3D tomography, the samples need to be imaged from many angles, which can exacerbate the problem.
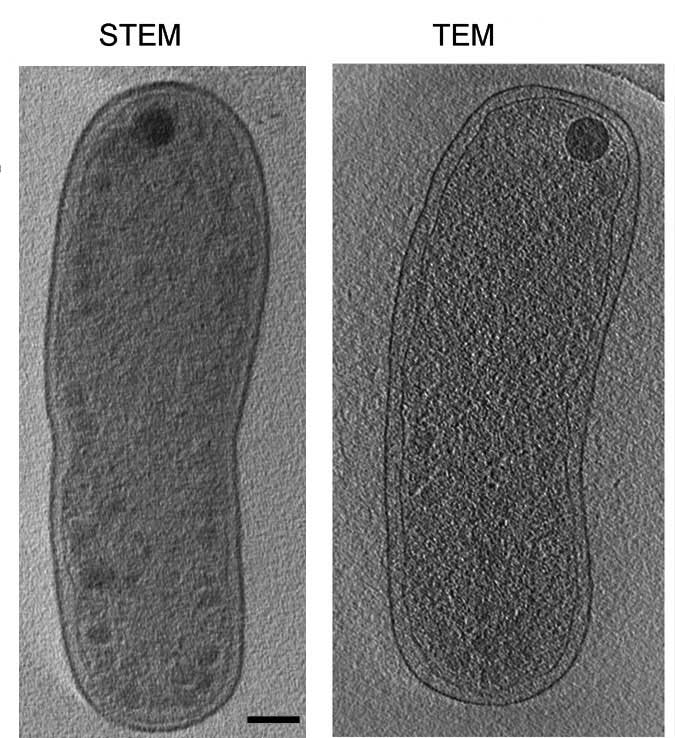
Conventional imaging methods that illuminate the entire sample area all at once are plagued by haze because of random scattering. If the sample is thick, a special filter is required, which can throw away 90% or more of the already-weak imaging signal. The team found that by configuring the transmission electron microscope (TEM) to scan the sample point by point – a method that is standard in materials science but not in biology – the haziness could be neutralized without losing signal. “It's a bit like searching at night,” says Elbaum. “Due to the way that electrons interact with matter, unstained biological specimens are always ‘foggy.' When it's clear, a floodlamp is fine, but in a fog it's better to search with a focused flashlight.” The TEM detectors were also adjusted so as to collect their signals at unusually low angles. This aspect of the new method – recording the weakly scattered electrons at sufficiently low angles – is not at all standard.
The scientists were able to “hack” the available hardware to meet their needs, and the results were striking: The unconventional configuration revealed images with superb contrast. Such an outcome had previously been considered so unlikely that this experimental setup had not until now been attempted. The scanning beam method allows the use of samples two to three times thicker than had previously been possible.
Indeed, to test this newly adapted method, the team imaged such “gigantic” (for a conventional TEM) specimens as whole bacterial cells, as well as human tissue culture cells. The improvements surprised even the scientists: High-contrast, good quality three-dimensional images were being produced that were much better and clearer than those obtained by the traditional TEM method.
This new technique, recently published in
Nature Methods, should broaden the applicability and accessibility of transmission electron microscopes for biological research. Wolf: “To extract the best results from three-dimensional TEM imaging of ultra-rapidly cooled specimens, very expensive equipment is required. In comparison, the hardware for STEM capability is a simple add-on to an existing modern TEM, which offers a path into the field for many researchers.” The scientists plan to improve upon this method even further, including designing new tools to optimize the data collection.
Elbaum: “The multidisciplinary nature of the Weizmann Institute has frequently been instrumental in the pursuit of unconventional ideas and technology, and the Electron Microscopy Unit, supported by the Moskowitz Center for Nano and Bio Nano Imaging, is really a case in point. Few places in the world would enable such close encounters of scientists with different areas of expertise, let alone give them the freedom to focus together on a risky project. At Weizmann it was entirely natural.”
Prof. Michael Elbaum’s research is supported by the Irving and Cherna Moskowitz Center for Nano and Bio Nano Imaging, which he heads; the Louis and Fannie Tolz Collaborative Research Project; and Sharon Zuckerman, Canada.