Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:

In a discovery filled with surprises, Institute scientists have created a new type of nanotube made of gold, silver and other metals. Made at room temperature - a first time achievement - the tubes exhibit unique electrical and optical properties, and may lead to a variety of applications in medicine, industry and security systems.
Nanotubes are among the most promising materials of nanoscience, a rapidly growing field aimed at creating novel materials and structures by manipulating matter on the tiniest of scales - atom by atom, molecule by molecule. (Nanos, the Greek for dwarf, is one billionth, so a nano-meter is one billionth of a meter - or roughly one hundred-thousandth the width of a human hair.)
The first nanotubes, discovered in 1991, were made of carbon and captured the attention of scientists worldwide when, despite their incredibly small dimensions, they proved to be the strongest material ever made (see box).
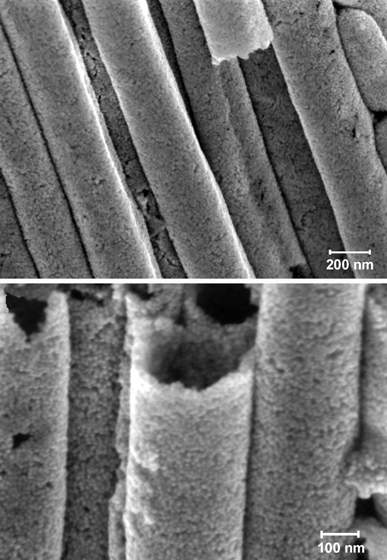
The new nanotube created at the Institute lacks the mechanical strength of carbon nanotubes. Its advantages lie instead in its use of nanoparticles made of gold, silver and other metals as building blocks. This makes it possible to tailor the tube’s properties to diverse functions according to the nature of the nanoparticles chosen. These nanoparticles can also serve as a scaffold for various add-ons, such as semiconducting or polymeric materials, thus further expanding the tubes’ available properties.
The study, published in Angewandte Chemie, was performed by Prof. Israel Rubinstein, Dr. Alexander Vaskevich, post-doctoral associate Dr. Michal Lahav and Ph.D. student Tali Sehayek - all of the Institute’s Materials and Interfaces Department.
The scientists had started out with a totally different target - to create nanosized templates for studying how biological molecules pass through different membranes. “We were amazed when we discovered the beautifully formed nanotubes,” says Rubinstein. “The construction of nanotubes out of nanoparticles is unprecedented, and,” he adds smiling, “the twist is that we’re not yet sure how this happens, which, of course, is one of the fun things about science.”
What is clear is the specific scheme that led to the tubes’ formation. The team started out with an aluminum oxide template with nanosized pores, which they modified chemically to make it readily connect to gold or silver nanoparticles. When a solution containing the nanoparticles was poured through (each only 14 nanometers in diameter), the particles bonded to both the template and to one another, creating multi-layered nanotubes in the template pores. In the final step, the template can be dissolved, leaving an assembly of free-standing, solid nanotubes. “We expected the nanoparticles to bind to the template - that had been done before; but we did not expect them to bind to one another, creating the tubes,” says Rubinstein.
The resulting tubes are porous and have a high surface area, distinct optical properties and electrical conductivity. Collectively, the tubes’ unusual properties may enable the design of new catalysts as well as sensors capable of detecting diverse substances present in minuscule amounts. A key feature of their success would be the ability, due to the tube’s room-temperature production, to add on biological molecules that would otherwise be destroyed by high production temperatures. These would then perform their natural function of recognizing other molecules in nature, in a key-fits-lock manner. Other tube applications might include lab-on-a-chip systems used in biotechnology, such as DNA chips that detect genetic mutations or evaluate drug performance. Yeda, the Institute’s technology transfer arm, has filed a patent application for the tubes.
While major hurdles remain, scientists believe that the products of nanoscience might change our future. Carbon nanotubes, for instance, are already a favorite with researchers worldwide. Researchers estimate that a fiber about the width of a human hair made of these carbon tubes could support around 2 tons. The first experiment demonstrating the strength of carbon nanotubes was performed here at the Institute by Prof. Daniel Wagner. The tubes’ phenomenal strength (over 100 times stronger than steel) has triggered research targeting applications that run the gamut from classic engineering quests, such as longer bridges and taller buildings, to science-fiction- like missions, including nanotube cables that would tether a satellite in orbit or even make possible “space elevators” carrying people or equipment into outer space.
Look for Rubinstein and his team outside of the lab and you might be surprised. Rubinstein, for instance, has a black belt in karate, having picked up the sport at age 45; Vaskevich (Sasha) spends his downtime trekking through mountains and art museums; for Michal Lahav, currently pursuing a postdoc at Harvard University, time-off is best spent deep-water diving in the Red Sea’s world-famous reefs; and roaming through the jungles of Peru or the temples of Thailand is how Ph.D. student Tali Sehayek chooses to relax.
Prof. Rubinstein’s research is supported by the Clore Center for Biological Physics; the Fritz Haber Center for Physical Chemistry; the Angel Faivovich Foundation for Ecological Studies; the Philip M. Klutznick Fund for Research; the Edward D. and Anna Mitchell Family Foundation; and Minerva Stiftung Gesellschaft fuer die Forschung m.b.H.
Prof. Wagner is the incumbent of the Livio Norzi Professorial Chair.