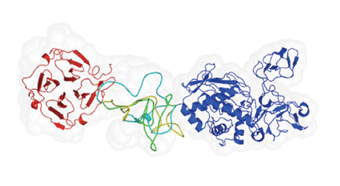
When cancer cells metastasize or tissues become damaged through inflammation, it’s likely that enzymes called matrix metalloproteinases (MMPs) are involved. This family of enzymes cuts through various bodily materials, including the tough collagen fibers that hold our tissues together.
One member of the family in particular – MMP-9 – is often produced by migrating cancer cells and in certain autoimmune diseases, and scientists have long believed that finding a way to inhibit its activities might be useful for treating these diseases. A team led by Prof. Irit Sagi of the Structural Biology Department in the Faculty of Chemistry has now employed an unconventional combination of techniques to reveal the structure of the entire MMP-9 protein. The team included Gabriel Rosenblum of the Structural Biology Department, Drs. Phillippe Van den Steen and Ghislain Opdenakker of the University of Leuven, Belgium, and Dr. Sidney Cohen of the Institute’s Chemical Research Support.
Their findings revealed a linker whose extreme flexibility and contortions “would impress even a swami yogi,” in the words of a scientific reviewer. The distinctive MMP-9 linker may turn out to be its Achilles’ heel: The team has already designed a molecule that binds directly to this domain to neutralize its activity, and Yeda, the business arm of the Weizmann Institute, has applied for a patent for this molecule.
Prof. Irit Sagi’s research is supported by the Avron-Wilstaetter Minerva Center; Mr. and Mrs. Michael Ambach, Boca Raton, FL; and the estate of David Turner. Prof. Sagi is the incumbent of the Maurizio Pontecorvo Professorial Chair.