Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Hundreds of millions of years have passed since we split off from our common ancestor with bacteria, but we still share a kind of immune system with them. This was the finding of research in the group of Prof. Rotem Sorek of the Weizmann Institute of Science, recently reported in Nature. The findings of this study provide some clues as to the evolution of immune responses, and they may lead to new insights into treating autoimmune disease and even cancer.
Sorek, a member of the Institute’s Molecular Genetics Department, explains that bacteria must continually protect themselves from viruses called phages that infect them. The most famous bacterial immune system is CRISPR, the gene-editor par excellence that can insert foreign DNA into its own genome, and which is today used in biology labs for this purpose. But there are dozens of other such bacterial immune systems – many of them discovered in Sorek’s lab -- and each has the potential to give us new tools to fight disease.
Sorek and his team were intrigued last year when a molecule called cyclic GMP-AMP (cGAMP) was discovered in bacteria. This molecule – made of two nucleotides, A and G – had first been identified in human cells. Less than a decade ago it was found that cGAMP is part of an immune system carried in every one of our cells; each cell can activate it independently. The basic principle of the cells’ autonomous immune system is simple: Any double-stranded DNA – human or viral -- found outside the cell nucleus is “illegal” and considered potentially dangerous. Then several things occur: A virus-fighting molecule called interferon is produced and exported to the rest of the immune system, and a protein in the cell starts joining together pairs of A and G nucleotides to make cGAMP, which serves as a sort of indicator of the threat and directs further action.
But it was not clear why bacteria would have cGAMP: They have DNA like ours but lack a nucleus, so the “DNA in the wrong place” rule could not apply here.

The first clue that it might indeed be part of an immune response came when the research team located the genes responsible for bacterial cGAMP in “defense islands” – regions of the genome in which immune-related genes tend to cluster. Eventually they uncovered four genes that work together in this system.
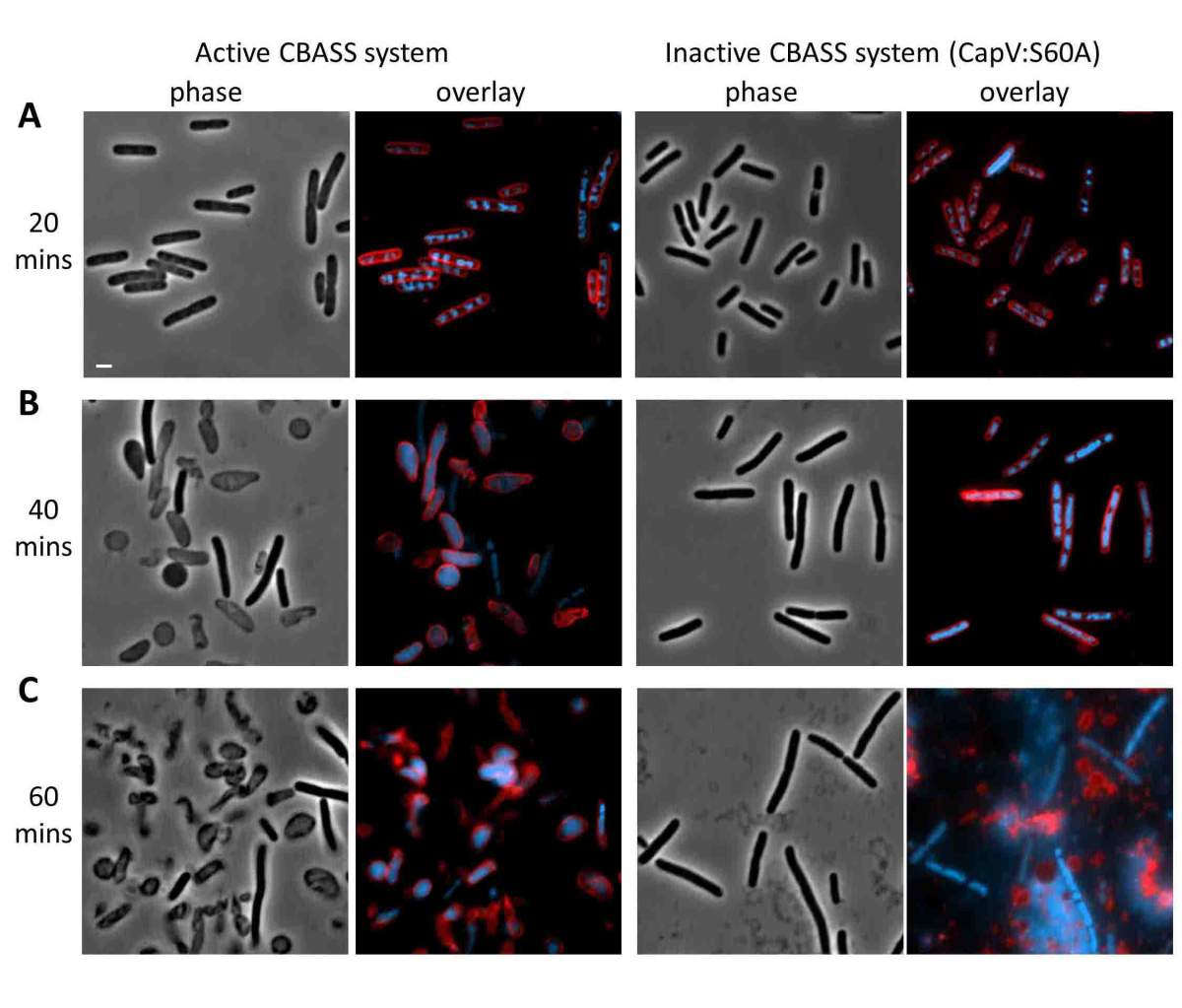
To further investigate whether the cGAMP genes are, indeed, immune-related, the researchers took these four genes from their native bacteria and inserted them into a bacterium that does not have this system – E. coli. They then infected the E. coli with phages. The scientists found that the bacterial colonies in their lab dishes grew much better and were up to a thousand times more phage resistant when the four-gene system was present, and that the system appeared to protect against a broad range of phages. In other words, the bacterial cells, like human ones, employ a cGAMP to quickly identify a potential threat and act.
The research showed that this system protects bacteria by inducing suicide. Once thought to be the sole province of cells in multi-celled organisms, such altruistic cell death is now known to be hard-wired into many single-celled organisms as well. When Sorek and his team directly infected their engineered cells with phages and checked them some forty minutes later, the cells had died. When they opened the cells and looked inside, they found a pattern of cGAMP accumulation peaking at cell death. Forty minutes is about half the time needed for a virus to replicate itself inside a bacterium, so the death of a few cells can protect a colony from further infection. Cell death is initiated by activating proteins near the cell’s edge that eat away the cell membrane. At the same time, cGAMP production enables the cell to continually sense the viral load so it can take a step back if it manages to deal with the infection by less drastic means.
The differences between the systems appears to lie in the preferred method of suicide
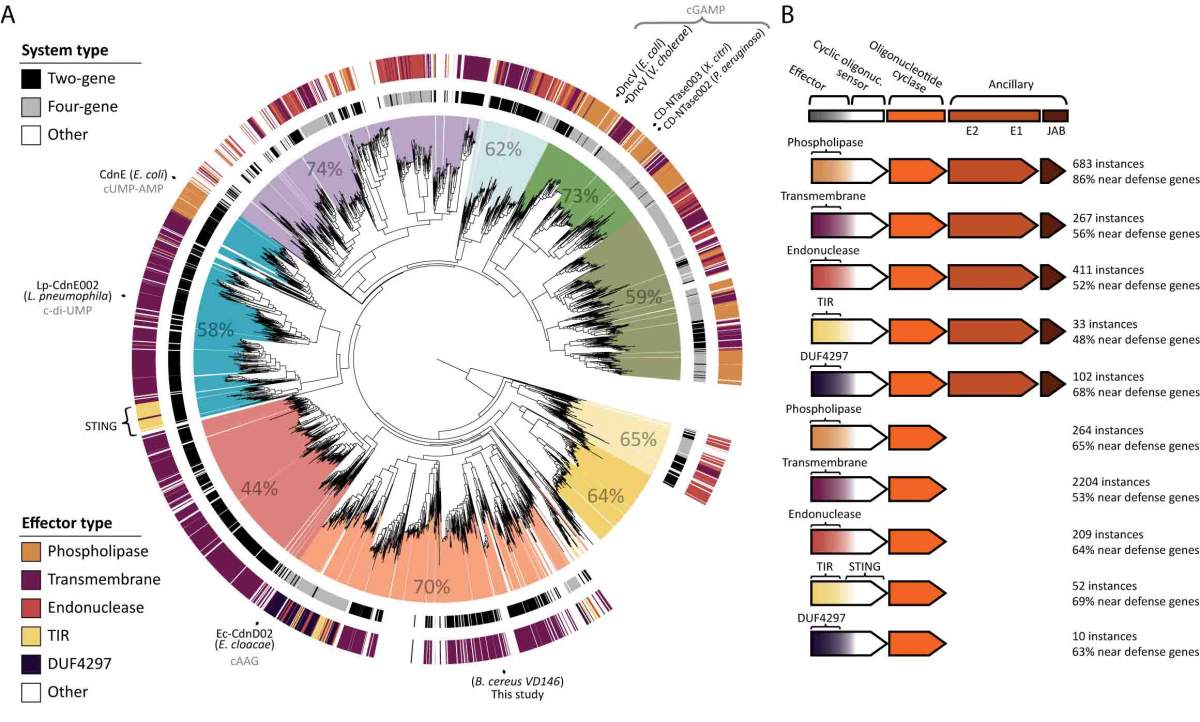
As Sorek and his team were investigating this primitive immune system, a group at Harvard University discovered that bacteria can harbor a variety of cyclic nucleotides, in addition to cGAMP. Each has its own combination – two U’s instead of cGAMP’s A and G, for example. The Weizmann Institute group dubbed the family by a new acronym – CBASS – and mapped the relations between family members. They then conducted experiments on one of them, finding that it, like cGAMP, is part of an anti-phage system. “All of these cyclic nucleotide systems are likely immune defense systems,” says Sorek. The differences between the systems appears to lie in the preferred method of suicide: One drills holes in the membrane, while another cleaves the bacterial DNA, etc.
“The autonomous immune systems in our cells go all the way back to a time when all creatures were single-celled and lacked nuclei,” says Sorek. So the first eukaryote – an organism that gained a cell nucleus when two cells of different types merged – already came equipped with this anti-viral mechanism, which has survived to this day.”
The research team identified the CBASS immune system that is genetically closest to that found in our cells, and they think this finding could be relevant to human health. The human version has been implicated in an autoimmune disease, in which it is overactive; while cancer cells are thought to inhibit this system, keeping cells with dangerously leaky nuclei alive. “We know that certain phages manage to evade the CBASS immune systems. If we can learn from them how to turn the immune response up or down, we might be able to use this insight to treat disease,” says Sorek.
Prof. Rotem Sorek's research is supported by the David and Fela Shapell Family Foundation INCPM Fund for Preclinical Studies; the Knell Family Center for Microbiology; the Sagol Weizmann-MIT Bridge Program; the Schwartz/Reisman Collaborative Science Program; the Yotam Project; and the European Research Council.