Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Like the children in Neverland who never grow older, we have cells in our bodies that retain their youth, even as our bodies age. These cells – the adult stem cells – work continuously to renew our body’s cells; none work harder than the blood stem cells, which endlessly differentiate to produce some 500 billion blood cells a day. But researchers who study these cells have found that the instant they are removed from their “Neverland” within the bone marrow and grown in cell culture, they lose their youthful properties, differentiating entirely into adult blood cells within days. New research at the Weizmann Institute of Science has now revealed a mechanism that instigates differentiation in blood stem cells. The findings of this study, which were recently published in Nature Cell Biology, may point to ways of growing stem cells in culture for transplant or even ways of targeting adult blood cells, for example, in cancer treatments without harming the source – the blood stem cells.
Prof. Jacob Hanna and his group in the Institute’s Molecular Genetics Department are experts on a different type of stem cell: the embryonic stem cell ‒ the short-lived cell found only in the earliest embryo that can differentiate into any cell type. Adult stem cells, in contrast, stay with us our entire lives, but they can differentiate only into a limited number of adult cell types. Quite a large body of research is focused on the blood stem cells, which exist only in very small numbers but are highly sought after for bone marrow transplants; and much of this research has focused either on mechanisms that can give them the push to differentiate or, conversely, on those that may be used to preserve their “stemness.” “With adult stem cells, this is nearly impossible,” says Hanna. “Outside the body, they are actually less stable than the embryonic kind, and they quickly lose their unique properties.”
In their previous research, Hanna and his group had discovered that an enzyme called Mettl3 pushed the embryonic stem cells in their lab to differentiate. This enzyme works by a process known as RNA methylation – that is, attaching a methyl group to a specific location on a strand of messenger RNA, thus altering the instructions carried on the strand. The group found that deleting the Mettl3 enzyme in mouse embryos brought a halt to differentiation and, indeed to the future of those embryos.

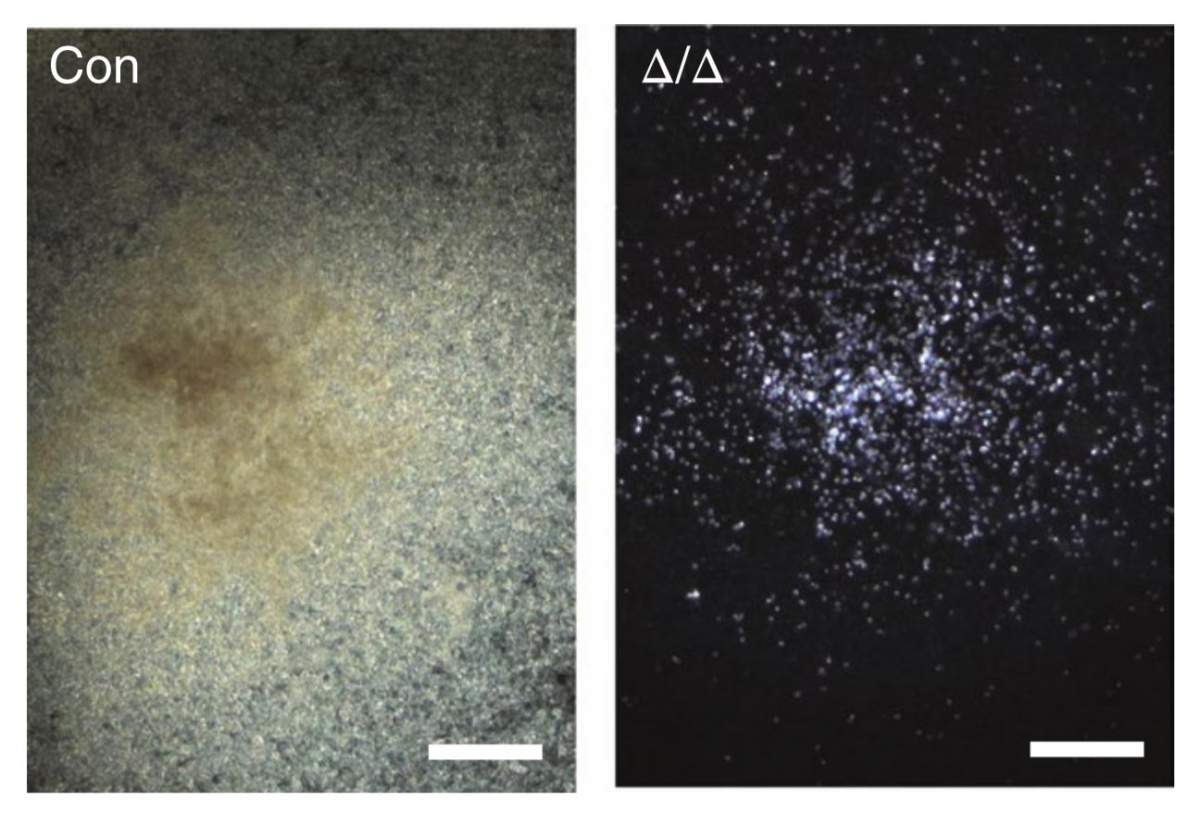
Does Mettl3 play a similar role in adult stem cells? To investigate, Hanna and his group joined forces with those of Prof. Lei Ding of Columbia University Medical Center. They created mice in which the enzyme could be selectively deleted in the blood stem cells alone. “Without the enzyme, no adult blood cells were produced and the mice became anemic. Surprisingly, the blood stem cells continued to live and divide as before. They just stopped differentiating,” says Hanna.
To follow up, the researchers uncovered the entire mechanism for initiating stem cell differentiation: After stopping the activity of Mettl3, and with it messenger RNA methylation, the levels of another protein known as Myc dropped precipitously. When the researchers restored Myc levels in the mice, blood cell differentiation was restored as well. The researchers noted that this mechanism was limited to the stem cells – not to the various cells that are “on their way” to becoming adult cells – a finding that further suggests this mechanism could be used to specifically manipulate the stem cell state.
The blood stem cells continued to live and divide as before. They just stopped differentiating.
Understanding this mechanism may, in the future, lead to techniques for culturing blood stem cells in large enough quantities that they can be transplanted into the bone marrow to treat leukemia or other cancers. But they also raise some other tantalizing possibilities. For example, such stem cells might undergo gene therapy before being inserted into the bone marrow. Or blood cell methylation levels could be lowered while administering chemotherapy treatments that kill blood cells, so these would finish off cells circulating in the blood stream but leave the crucial supply of stem cells alone. Since several pharmaceutical companies are already developing compounds to inhibit methylation – for other purposes – Hanna is hopeful this latter application will find its way to the treatment of cancer sooner rather than later.
Prof. Jacob Hanna's research is supported by Pascal and Ilana Mantoux; the Nella and Leon Benoziyo Center for Neurological Diseases; the David and Fela Shapell Family Center for Genetic Disorders Research; the Kekst Family Institute for Medical Genetics; the Helen and Martin Kimmel Institute for Stem Cell Research; the Helen and Martin Kimmel Award for Innovative Investigation; the Edmond de Rothschild Foundations; the Zantker Charitable Foundation; Rachel Peles; the estate of Zvia Zeroni; and the European Research Council.