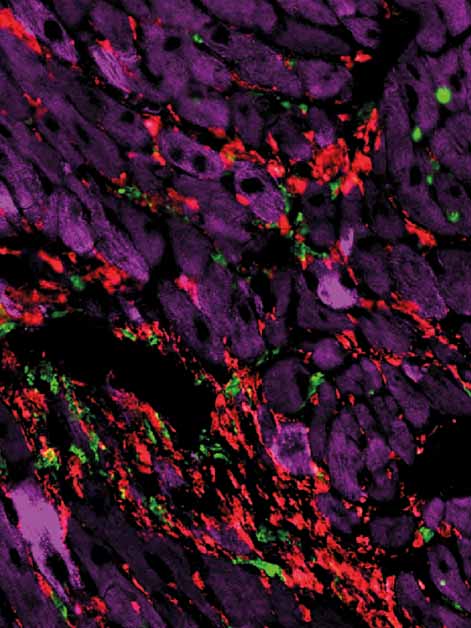
Not all scars are created equal. That’s the conclusion of a new study by Weizmann Institute of Science researchers: They found that two distinct types of scars, referred to as “hot” and “cold,” can form in the diseased heart, and that these two types call for entirely different treatments. As reported today in Cell Systems, the Weizmann study may lead to innovative therapies for preventing or treating heart diseases, and it opens a new line of research on fibrosis – the development of scar tissue in response to injury or during aging – in a variety of other organs.
The study began as a neighborhood collaboration. Prof. Eldad Tzahor, whose research focuses mainly on regeneration of the heart, learned about a mathematical model created in the lab next door, which is headed by Prof. Uri Alon. Alon’s model suggested classifying scars by the presence of two types of cells in the scar tissue: collagen-producing fibroblasts, which create fibrous scaffolding, and immune cells called macrophages.

“At first, it seemed too simple – after all, we all know how complex biological systems really are,” Tzahor recalls. “But I was intrigued by the idea, and I’d long wanted to collaborate with Uri. I suggested that we try applying his model to the injured heart.”
The heart is a pump that cannot function if it has a hole, so it immediately starts repairing any damage, such as that resulting from a heart attack. Since heart muscle cells don’t regenerate, the repair consists of cobbling together a scar tissue patch. The problem is that the scar can invade healthy heart tissue in the days or weeks following the heart attack, interfering with normal heart function. Scar tissue, while it plugs the “hole,” does not contract effectively, and as the severely scarred heart muscle carries on with pumping as best it can, many patients eventually develop chronic heart failure.
""Hot and cold fibrosis are two distinct cardiac diseases. This suggests that drug development might have to proceed along two different trajectories"
Currently there is no effective treatment for cardiac scarring. Therefore, preventing or reducing fibrosis is a pressing medical need. Yet despite the seeming simplicity of scar tissue, fibrosis is, in fact, an extremely complex process involving numerous cell types and signaling molecules.
Guided by Picasso
“In trying to cut through this complexity, we derived inspiration from Picasso,” says Shoval Miyara, Tzahor’s and Alon’s joint PhD student, who led the project together with Dr. Miri Adler of the Hebrew University of Jerusalem, a former student of Alon. Miyara refers to the analogy that is sometimes drawn between mathematical models of biological systems and certain abstract art – something like Pablo Picasso’s bull that is immediately recognizable even though drawn with just a few lines. “We wanted to get to the essence of fibrosis in the heart,” Miyara explains.
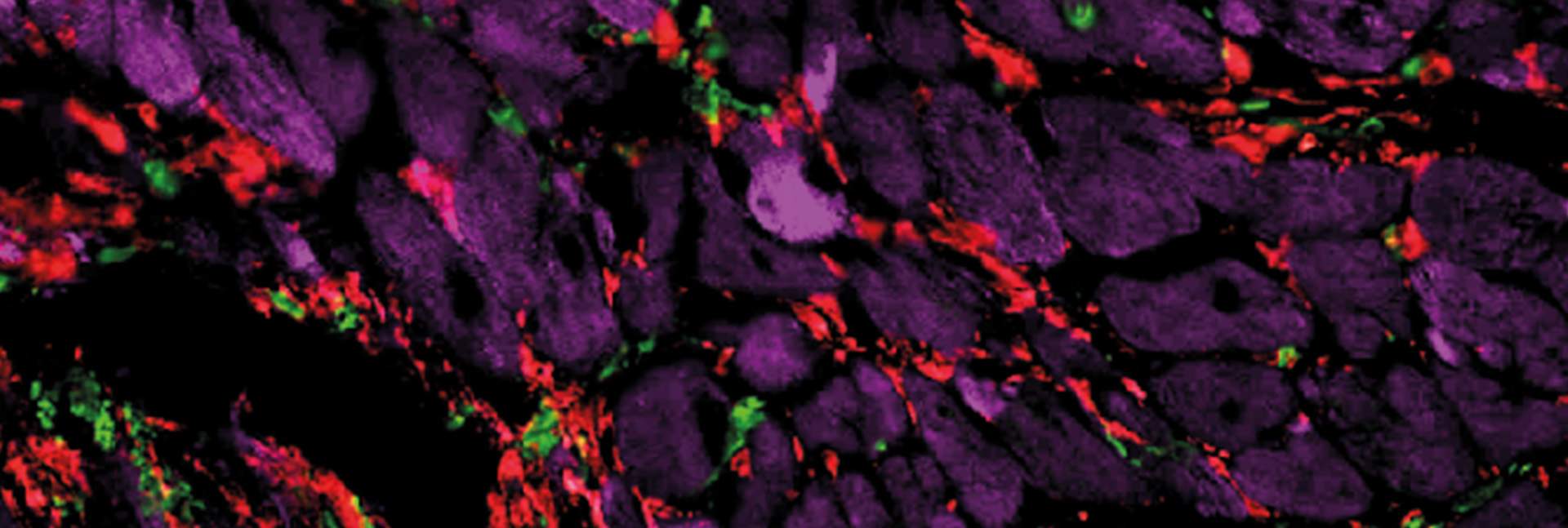
When he and colleagues applied Alon’s mathematical model to the injured heart, the model’s predictions departed from the classical picture, which treats all heart scarring as the same. The predictions said that in some scars, in addition to myofibroblasts – the active fibroblasts present in the injured heart – there are many immune cells called macrophages. The scarring defined by the presence of these two cell populations – which are dependent on an exchange of sustaining molecules among them – was named hot fibrosis, in a metaphoric reference to the heat and inflammation often associated with an immune response. In contrast, other scars, dubbed cold fibrosis, would contain self-maintaining myofibroblasts but almost no macrophages. The model therefore suggested that the two types of fibrosis in the heart are governed by different mechanisms.
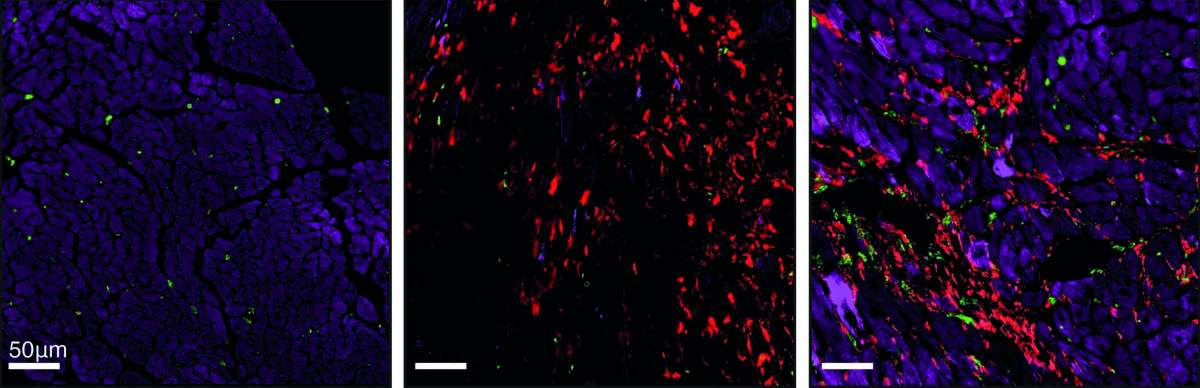
When Miyara and the team tested these predictions in cultured cells and in a genetically engineered mouse model they created for this study, they discovered that, indeed, chronic heart injury is characterized by hot fibrosis, whereas acute injury, such as a heart attack, leads to cold fibrosis. Although some immune cells are present immediately after a heart attack, within a few weeks hardly any macrophages remain in the scar tissue. That same picture emerged when the scientists tested heart tissue samples of human patients.
“Textbook microscopic images of injured heart muscles all look the same – they generally show collagen fibers making up the scar tissue,” Tzahor says. “But in fact, we showed that hot and cold fibrosis, driven by different biological mechanisms, must be treated differently.”
Targeting the scar makers
Focusing on the molecular processes governing cold fibrosis, Miyara and colleagues revealed the details of the loop-like mechanism in which myofibroblasts secrete molecules that spur further fibrosis. The researchers identified a key molecule in this loop: a protein called TIMP-1, which is known for several functions, but was for the first time shown in this study to work as a growth factor that facilitates the myofibroblast division and cardiac fibrosis. When the scientists blocked TIMP-1 in mice after a heart attack, the scar tissue that formed in their heart muscle was smaller than that which formed in untreated mice.

“We’ve shown that the TIMP-1 protein is a potential target for future drugs against fibrosis, but it’s clearly not the only one,” Miyara says. “Further research can reveal others, showing which ones could be most effectively targeted to prevent or minimize damage to the heart muscle after a heart attack.”
Sums up Tzahor: “We’ve found that hot and cold fibrosis of the heart are two distinct cardiac diseases. This suggests that drug development for these diseases might have to proceed along two different trajectories.”
Adds Alon: “This collaboration opened my heart to the fascinating biology of the body’s blood pump. I hope it will inspire new studies at the interface of mathematical models, basic biology and clinical needs. Future studies can test whether the concept of hot and cold fibrosis applies to scarring of other tissues, including those of the lung, kidney and liver, and to scars resulting from a variety of diseases, such as cancer and perhaps even stroke.”