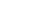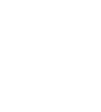Phages, viruses that attack bacteria, have a head and a tail. The head contains the phage’s genetic material and the tail is used to identify a potential host, that is, a bacterial cell into which it can inject this material. Once the injection is complete, the phage hijacks the bacterium’s cellular machinery and forces it to produce new copies of itself, which ultimately burst the cell and infect other bacteria in the colony. In a new study being published today in Nature, Weizmann Institute of Science researchers reveal a bacterial immune system that thwarts the phages’ plot by attaching a small protein molecule to their tails. The components of this new immune system are similar in structure to a human immunity mechanism, and they might help reveal how this mechanism works and how our own immune system has evolved.

The first antiphage defense mechanisms in bacteria were discovered in the 1960s, but only a handful of such mechanisms were known until recently. The most famous of these is CRISPR-Cas9, whose discovery led to a revolution in gene editing. In recent years, however, there has been a wave of new findings in the field, leading to the discovery of more than 150 new bacterial immune systems with varied modes of action. Many of these systems were identified using a method developed by Prof. Rotem Sorek of Weizmann’s Molecular Genetics Department.
Sorek’s method is based on a strikingly simple principle: Genes involved in bacterial immune mechanisms tend to cluster together in the bacterial genome, in areas known as “defense islands.” Researchers can therefore discover new immune systems by examining genes with unknown function that are located close to known defense islands. “In many of our studies, we have recognized components of bacterial immune systems that were familiar to us from extensively studied human immune mechanisms,” Sorek explains. “This suggests that the evolutionary source of a large part of our innate immune system comes from bacteria. Our new study provides further support for this idea.”
Ubiquitin on the shoulders of giants
In the 1970s, scientists uncovered a cellular control system capable of altering the structure and role of proteins, as well as their lifespan, by attaching a small protein called ubiquitin to them. Since ubiquitin’s discovery – for which professors Aaron Ciechanover, Avram Hershko and Irwin Rose were awarded the 2004 Nobel Prize in Chemistry – other scientists have revealed many similar systems, in which enzymes attach various small proteins to the target protein, thereby changing its destiny.
In the new study, researchers led by Dr. Jens Hör from Sorek’s laboratory discovered a new bacterial immune system that contains a ubiquitin-like protein with a structure similar to that of ISG15, one of the more mysterious proteins in the human immune system. ISG15 plays a role in the defense against different viruses, such as influenza and HIV, but how it performs its task is not entirely clear.
Studies suggest that the evolutionary source of a large part of our innate immune system comes from bacteria
Hör and colleagues found that, unlike other bacterial immune systems, the system they discovered did not prevent viruses from hijacking the cell and creating duplicates of themselves: Bacteria that encoded this immune system, once infected, died and produced new viral progeny. But these viruses were “sterile,” meaning that they could not infect additional bacteria, which led the researchers to conclude that the new immune system is somehow able to stop the virus from spreading to other cells in the colony.
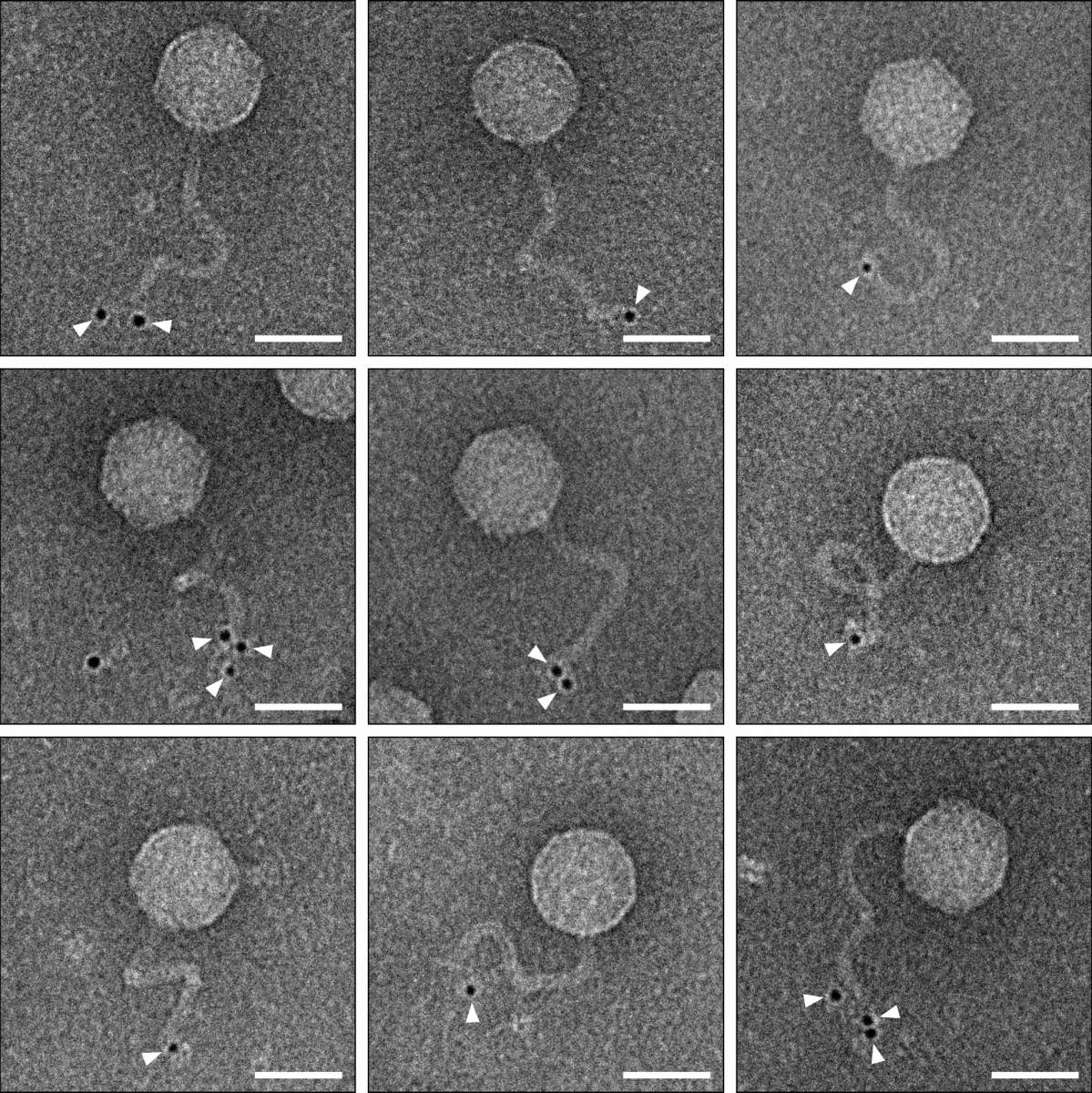
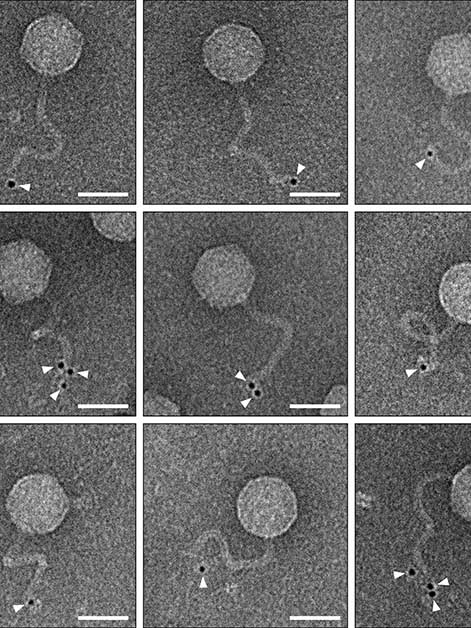
To understand how the duplicated viruses lose their ability to infect other cells and what role the new bacterial immune system plays in this, Sorek’s research team joined forces with Dr. Sharon Wolf, head of the Electron Microscopy Unit in Weizmann’s Chemical Research Support Department. The researchers labeled the ubiquitin-like protein at the heart of the new immune system with gold particles that are clearly visible under the microscope.
When they looked at the images of duplicated phages, they were astonished: The labeled protein located itself at the end of the viral tail, preventing the phages from using their tails to locate and infect new bacterial cells. The researchers believe that this new immune system is capable of recognizing the three-dimensional structure of the viral tail, which allows the system to work effectively against a wide variety of phages, as long as they have tails with a similar structure.