No matter how complex, all organisms – from the tiniest fruit fly to a human being – begin with a single cell. We know that this cell contains all the instructions for making every cell type in the body. The challenge is to uncover the overall plan laid out in the instruction book – the genome. What makes a head at one end and a tail at the other, a front and back, and, continuing through embryonic development, all the complex and diverse patterns that make up the living creature?
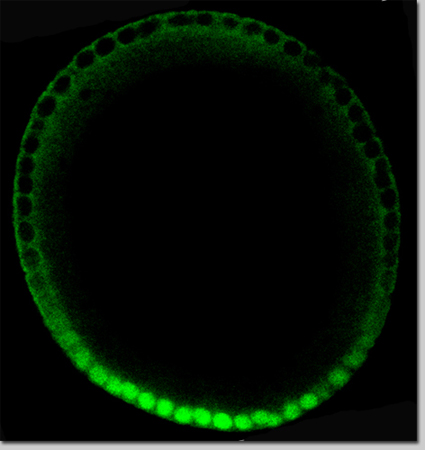
At formation, the fruit fly egg cell takes on rough coordinates that distinguish the head end from the tail, the back from the belly. During embryonic development, these rough coordinates gradually become refined, the entire pattern of the complete organism ultimately arising from this simple process. The first refinement takes place after fertilization, in the initial steps of embryogenesis: The rough markings turn into a sharp gradient with certain hormone-like proteins concentrated thickly in the center of the future belly and thinning out toward the edges of the region. In
research findings published in August in
Cell, Profs.
Naama Barkai and
Ben-Zion Shilo, and Michal Haskel Ittah, Danny Ben-Zvi, Merav Branski Arieli and Dr. Eyal Schejter of the Molecular Genetics Department revealed how the embryo carries out this step – one that will soon give rise to a wide spectrum of cell types along the belly/back axis. “The surprising thing,” says Shilo, “is that it accomplishes this feat with only a handful of components.”
Those components had been identified through genetic screening over the years, and the researchers knew that somehow, this limited set of players is sufficient to convert the rough coordinates of the egg to the refined pattern of the embryo. What’s more, the critical action takes place in a thin zone between two membranes – the egg membrane and an external outer membrane.
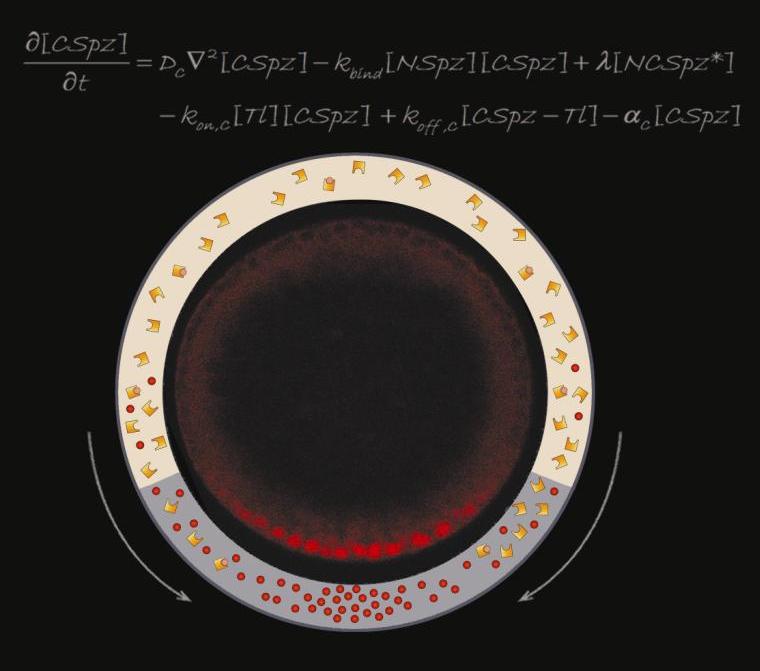
While the genes could tell the scientists which components were involved, they could not reveal how those components formed a working mechanism. For that, the team turned to computational approaches, creating theoretical models to see which would yield the gradients they observed in experiments. They looked for gradients that would hold and which would be reproducible even when the levels of individual components varied. “We know from previous experience that this constraint strongly restricts the space of possible mechanisms,” says Barkai. Although the number of elements, indeed, was limited, the researchers proposed mechanism is fairly elaborate, with at least one of those elements taking on several different forms. This protein, the hormone that triggers the response, is called “spaetzle” (so-named because when it is missing, the embryo becomes elongated, like the German noodle).
Spaetzle, in its inactive form, is found encircling the entire inner membrane of the early embryo; the enzyme that activates it – by cleaving it into two parts – is active only in the belly region. Once spaetzle is activated, the two parts can diffuse out of the belly region, and they can also recombine in a different form that can then be reactivated in a new way. This interplay – between the elements that are able to diffuse past the edges of the belly region and those that are confined to it – creates a situation in which active spaetzle proteins are concentrated towards the belly region, thus producing the activation gradient. Shilo: “We see, in our model, that the active form of the hormone is eventually driven in toward the center.”
With the mechanism proposed by the model in mind, the researchers turned back to experiments. They showed that the Spaetzle protein is indeed present in several distinct forms. When the team manipulated the genes for the elements in the model, they found they could direct the process of hormone concentration, even generating fruit fly embryos in which the belly region formed where the tail should be. These experiments convinced the researchers that the working model generated in computational approaches is truly valid.
The membrane receptors to which spaetzle binds are known as Toll receptors, and these were originally discovered in the 1980s in developing fruit fly embryos. The same Toll receptors were the subject of the 2011 Nobel Prize in Physiology or Medicine, but the prize was awarded for the discovery of a completely different role: They are vital for the innate immune response, our body’s first line of defense against invading pathogens. These receptors have been conserved over eons of evolution, and Shilo believes that the Toll receptor initially evolved for its crucial role in the immune system and was later co-opted for embryonic patterning in insects. Shilo: “It would be interesting to see whether the mechanism we discovered for concentrating the spaetzle proteins and creating a gradient might also apply to certain aspects of the innate immune response.”
Prof. Naama Barkai’s research is supported by the Azrieli Institute for Systems Biology, which she heads; the Helen and Martin Kimmel Award for Innovative Investigation; the Jeanne and Joseph Nissim Foundation for Life Sciences Research; Lorna Greenberg Scherzer, Canada; the Carolito Stiftung; the European Research Council; the estate of Hilda Jacoby-Schaerf; and the estate of John Hunter. Prof. Barkai is the incumbent of the Lorna Greenberg Scherzer Professorial Chair.
Prof. Ben-Zion Shilo’s research is supported by the Carolito Stiftung; the Jeanne and Joseph Nissim Foundation for Life Sciences Research; the Dr. Josef Cohn Minerva Center for Biomembrane Research; the estate of Georg Galai; and the Mary Ralph Designated Philanthropic Fund. Prof. Shilo is the incumbent of the Hilda and Cecil Lewis Professorial Chair of Molecular Genetics.