Are you a journalist? Please sign up here for our press releases
Subscribe to our monthly newsletter:
Billions of newborn neurons migrate in the embryonic brain, each searching for its exact destination in the cortex. Researchers at the Weizmann Institute of Science have discovered that during this challenging journey, the neurons receive help from a surprising quarter: They are assisted by immune system proteins, the same ones that later in life engage in protecting the body against disease-causing microbes.
“It makes sense for proteins to have multiple uses – after all, the number of proteins in the organism is limited, so evolution likes to use the same players in different games,” says research leader Prof. Orly Reiner of the Molecular Genetics Department. “Still, we didn’t expect to find immune proteins affecting the migration of neurons in the developing brain.”
Errors in neuronal migration have been observed in people with autism, schizophrenia and various intellectual disabilities; but how these errors came about was unknown. There is quite a bit of information about what happens inside the neurons during the migration, but not much is known about how this migration is affected by the external signals they need for direction.
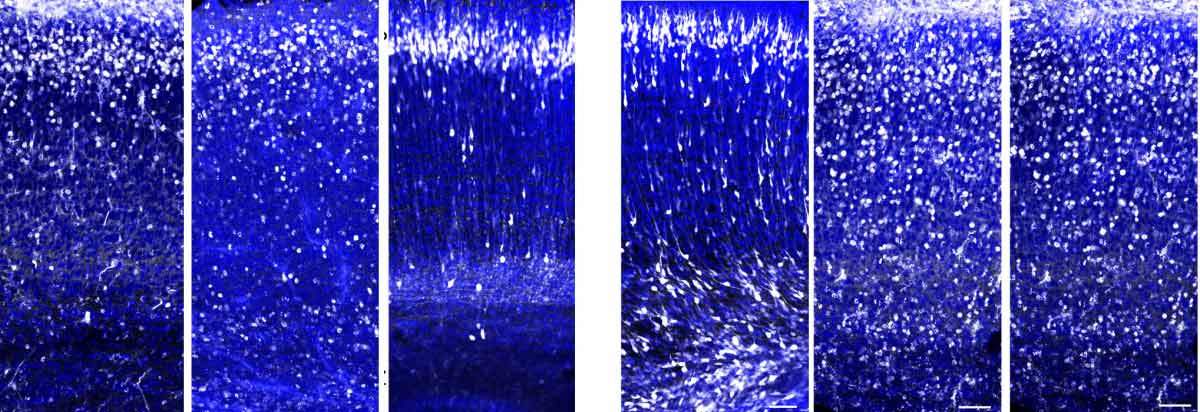
In the new Weizmann study reported in Nature Communications, postdoctoral fellow Dr. Anna Gorelik and other members of Reiner’s team examined some twenty proteins that had been identified in Reiner’s lab in an earlier study. These proteins could potentially be involved in the migration signaling because in the embryonic brain they float outside the neurons or are present on the surfaces of cells maturing into neurons. The researchers examined the genes and the proteins they encode, and to their surprise they discovered that one of them belongs to the so-called complement system: a system of small proteins that in the adult organism help clear microbes and damaged cells from the body.
The scientists then demonstrated that the complement system indeed plays a role in controlling the migration of emerging neurons. When they disabled (the technical term is “knocked out”) the gene for a protein called C3, a central component of this system, in the neurons of mouse embryos, they saw that these neurons failed to reach their normal destinations in the outer layer of the cortex; instead, they remained dispersed along the migration route.
We didn’t expect to find immune proteins affecting the migration of neurons in the developing brain
Next, the researchers, including Weizmann Institute staff scientists Drs. Tamar Sapir and Tsviya Olender of the Molecular Genetics Department, staff scientist Dr. Rebecca Haffner-Krausz of the Veterinary Resources Department and Prof. Trent M. Woodruff of the University of Queensland, Australia, showed that in the developing brain, C3 takes part in exactly the same chain of biochemical reactions as it does in the course of a certain type of immune response in the adult organism. Activation of this complement pathway causes the C3 to be cleaved, and the cleaved-off molecules subsequently activate complement receptors on the surfaces of neurons. The scientists managed to restore the proper migration of neurons in mice lacking the C3 by injecting the brain with small molecules that activate these complement receptors.

These findings may explain why people with a rare genetic disorder called 3MC syndrome – which is characterized by unusual facial features and other problems – often exhibit intellectual impairment. “Until now it was unclear how the mutated genes found in this syndrome, including those encoding proteins in the complement system, can lead to mental retardation,” says Reiner. “Our study provides a potential explanation: The mutations probably interfere with the appropriate migration of neurons during embryonic development.”
The study may also shed new light on autism. By consulting large databases of genetic information on autism, Reiner and her colleagues found that autistic individuals sometimes have newly formed mutations (that is, mutations that were not inherited) in genes encoding the complement system. “Past research had suggested that malfunctions of the immune system may contribute to symptoms of autism, but the underlying mechanism was unknown. We propose that mutations in the complement genes may be responsible for problems in neuronal migration. This revelation points toward a new direction of research into the neuronal mechanisms of autism.”
Prof. Orly Reiner's research is supported by the Yeda-Sela Center for Basic Research; the Lulu P. and David J. Levidow Fund for Alzheimers Diseases and Neuroscience Research; the Wohl Biology Endowment Fund; the Irving and Dorothy Rom Charitable Trust; and the Fritz Thyssen Stiftung. Prof. Reiner is the incumbent of the Bernstein-Mason Chair of Neurochemistry.